1Åm = 1×10 −10 m. The ångström is often used in natural sciences and technology to express the sizes of atoms, molecules, and microscopic biological structures, - ppt download
The wavelength of light of a particular colour is 5800 angstrom.express it in (a) nanometre and (b) metre.

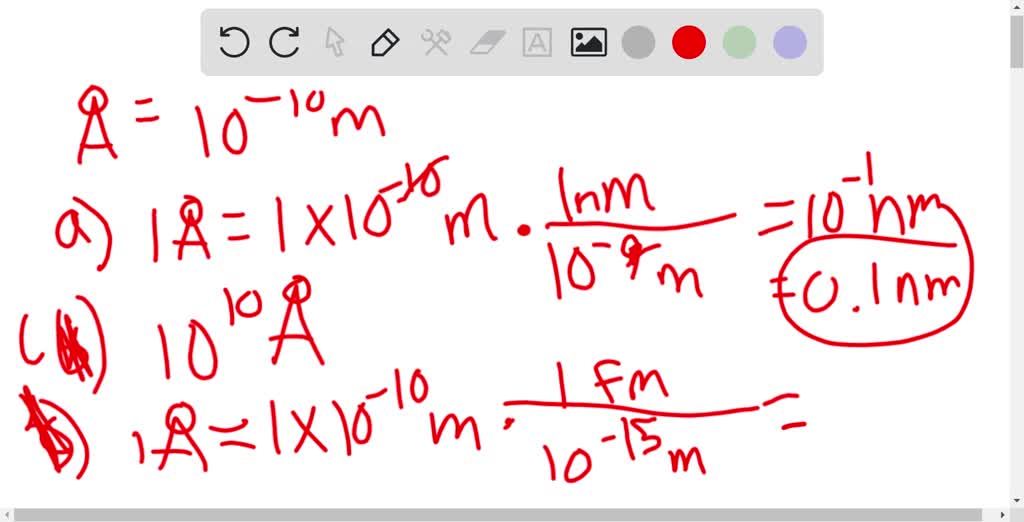
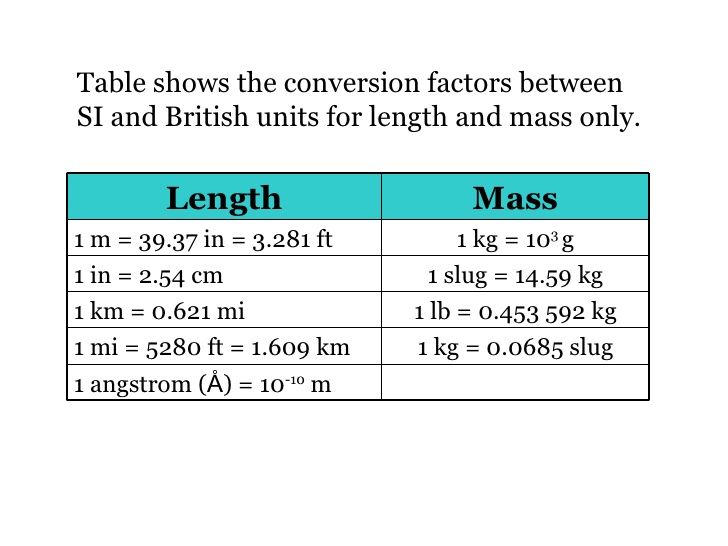
The unit length convenient on the atomic scale is known as an angstrom and is denoted by A: ( 1 A = 10^-10 m) . The size of a hydrogen atom is
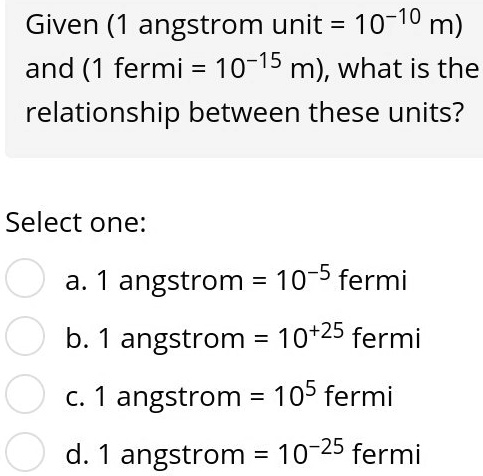
SOLVED: Given (1 angstrom unit = 10-10 m) and (1 fermi = 10-15 m); what is the relationship between these units? Select one: a, angstrom 10-5 fermi b. 1 angstrom 10+25 fermi CS 1 angstrom = 105 fermi d. 1 angstrom 10-25 fermi