
SOLVED: Write the net-ionic equation for the reaction between calcium carbonate and aqueous hydrochloric acid. First; write out each species as it exists in solution. Which of the reactants is (are) water-soluble?
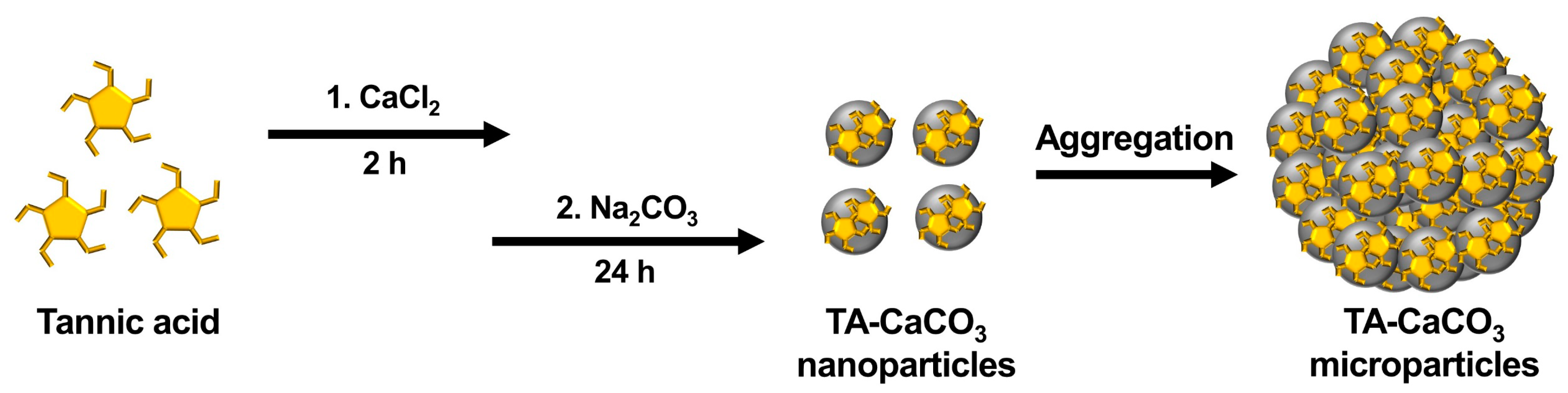
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects
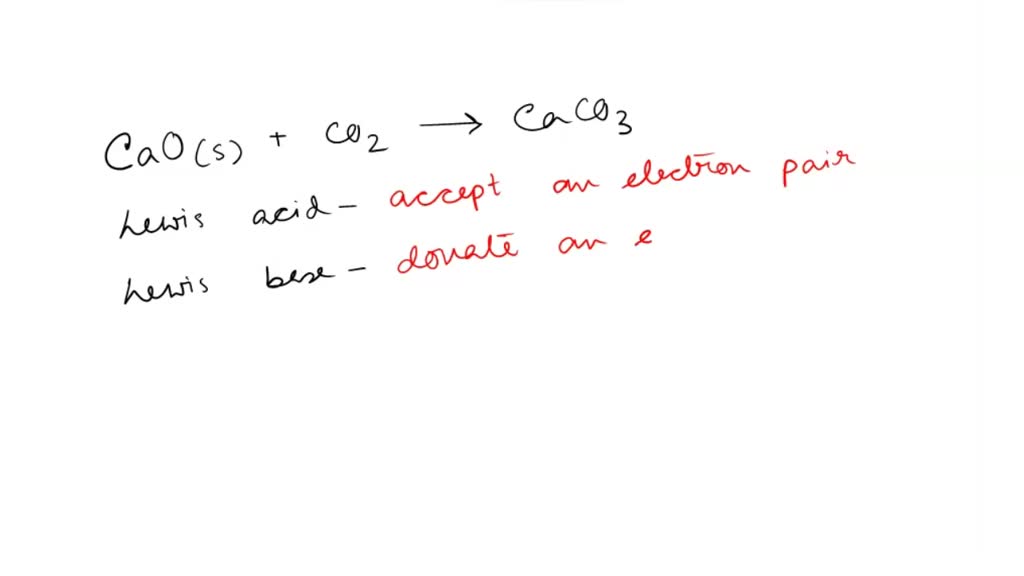
SOLVED: 'QUESTION 1 In the reaction: CaO(s) + C02(g) 5 CaCO3(s) 0A Ca2+acts as a Lewis acid and CO32- acts as a Lewis base 02-acts as a Lewis base and CO2 acts
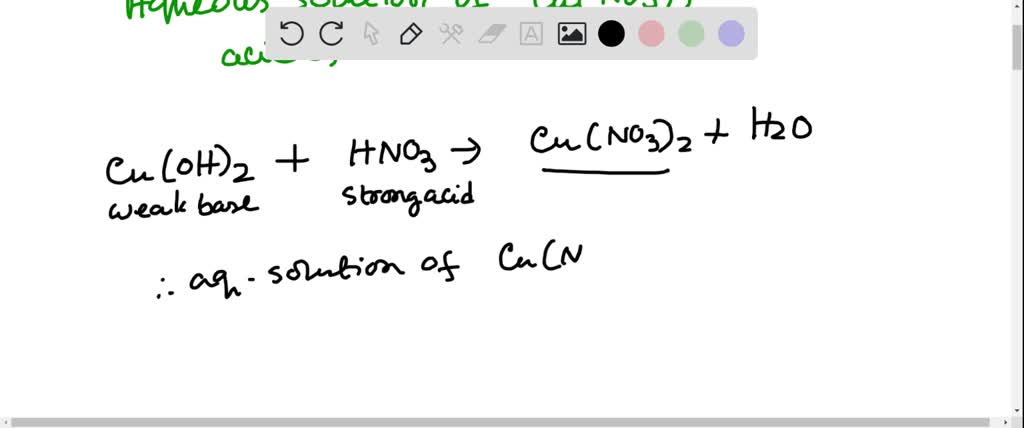
SOLVED: Solid nickel (III) nitrate, Ni(NO3)3, is dissolved in water. Is this solution acidic, basic, or neutral? Solid calcium carbonate, CaCO3, is dissolved in water. Is this solution acidic, basic, or neutral?

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

Acids, Bases and Salts Acids give up hydrogen ions (H+) in a water solution. Bases give up hydroxide ions (OH-) in a water solution. Mullis. - ppt video online download

CBSE Class 10 science term 1 Question Solution | parent acid and base of Calcium carbonate is....... - YouTube

![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/475685cc-4d58-4e74-a739-8cbc5d1c10f8/q8---parent-acid-and-base-of-calcium-carbonate---teachoo.jpg)







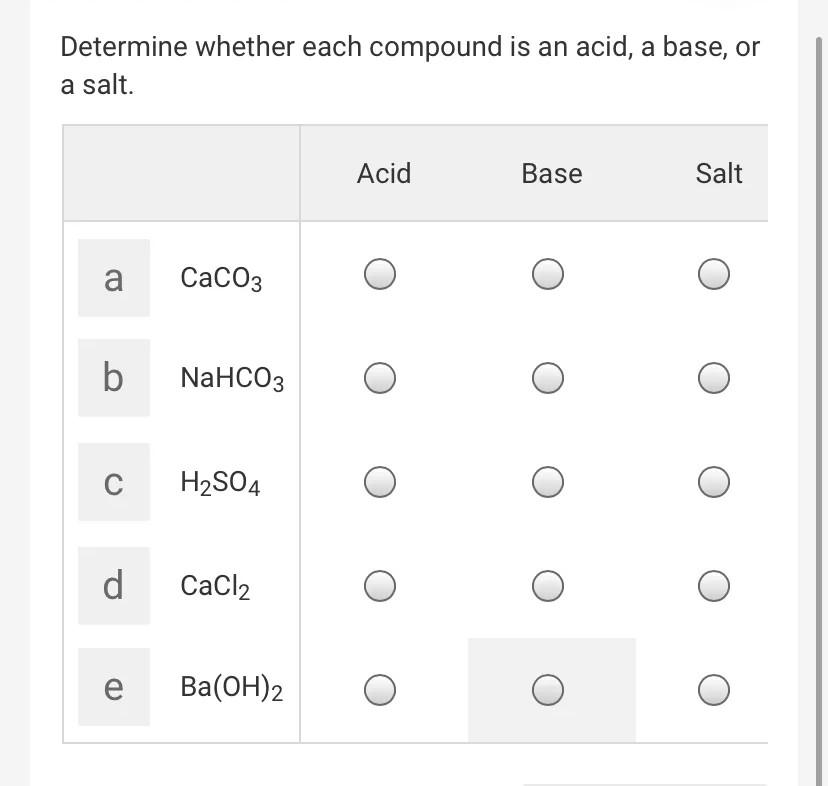
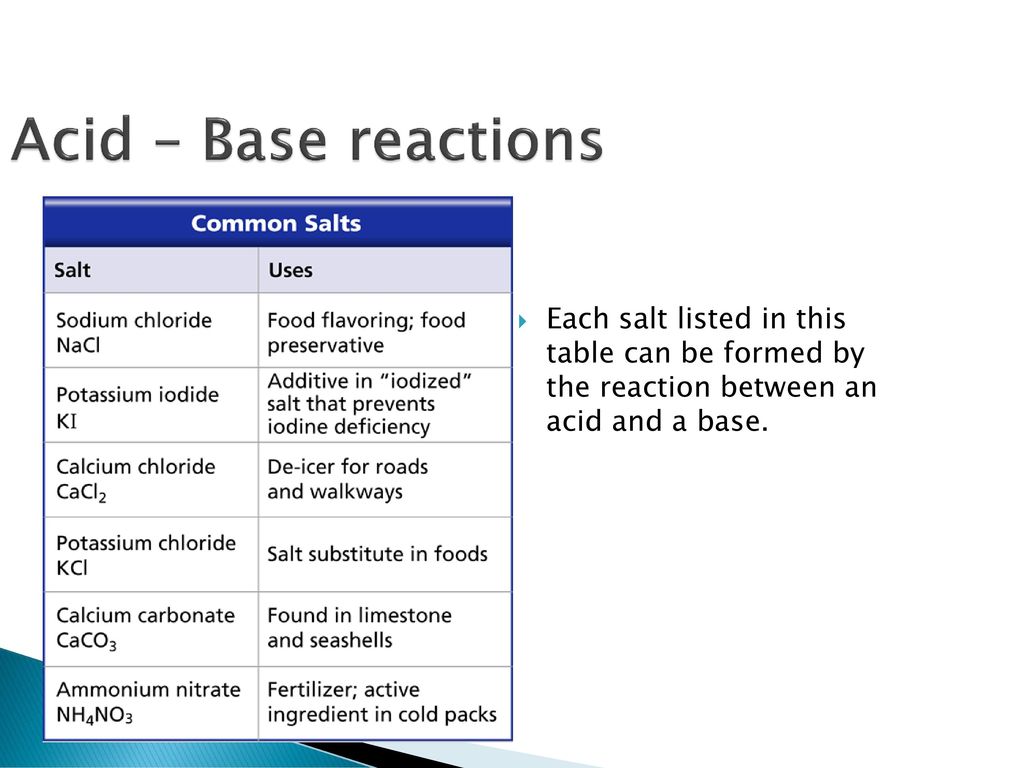
![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)
