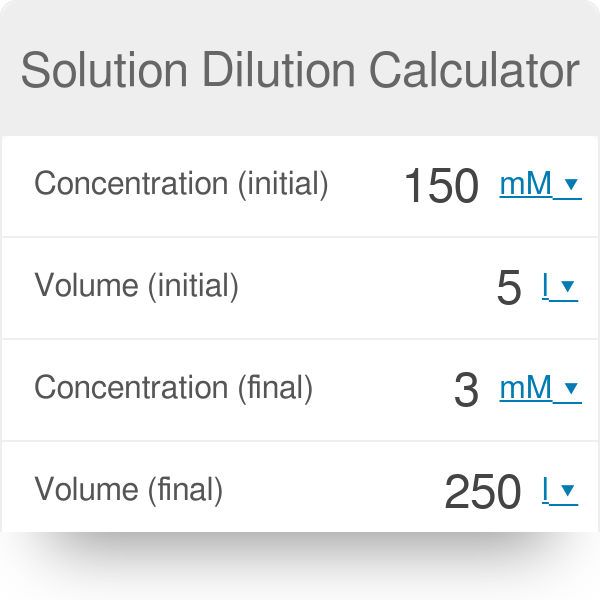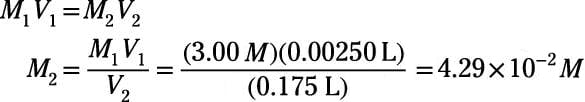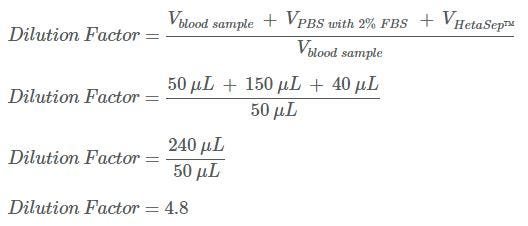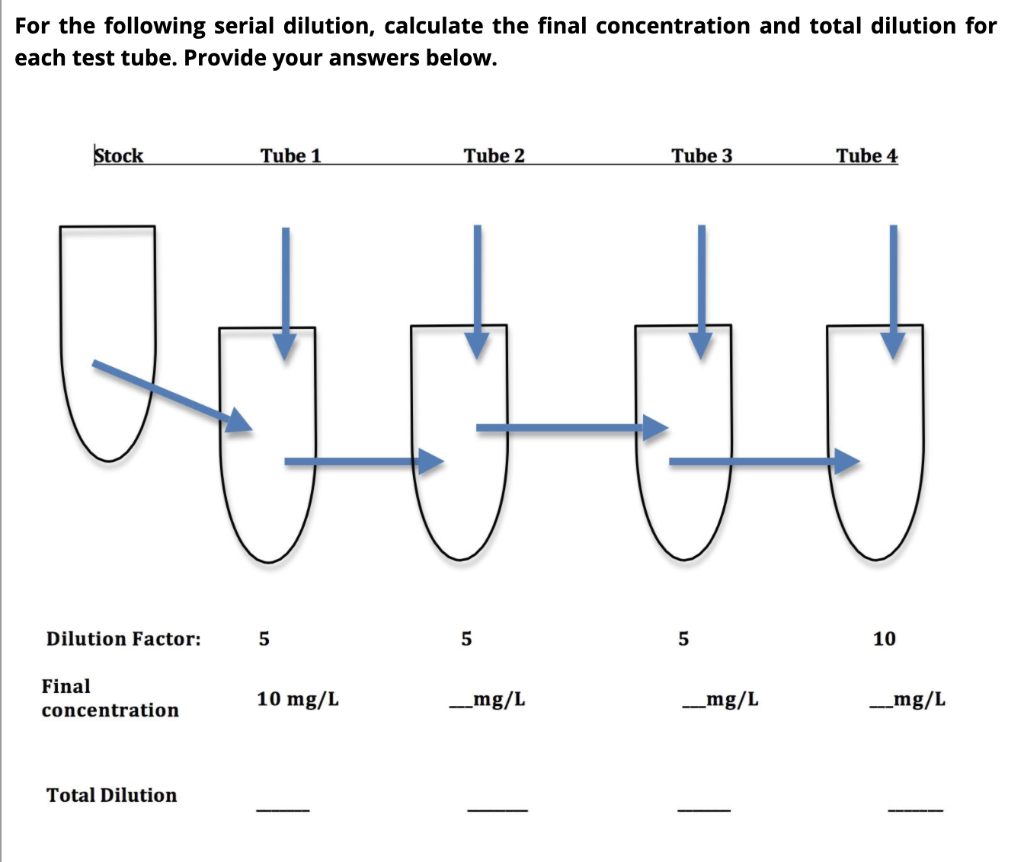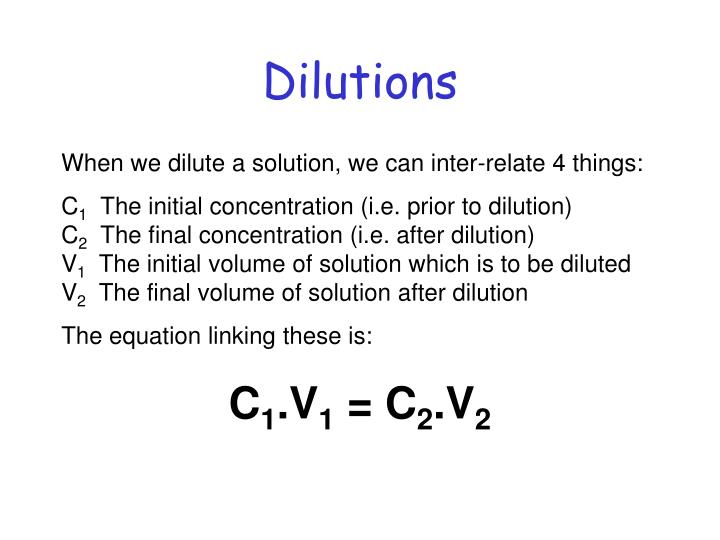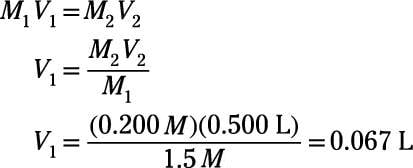
SOLVED: DILUTION AND CONCENTRATION When liquid medication of a given strength is diluted, its strength will be reduced. For example, 10 mL of a solution containing g of a substance has strength

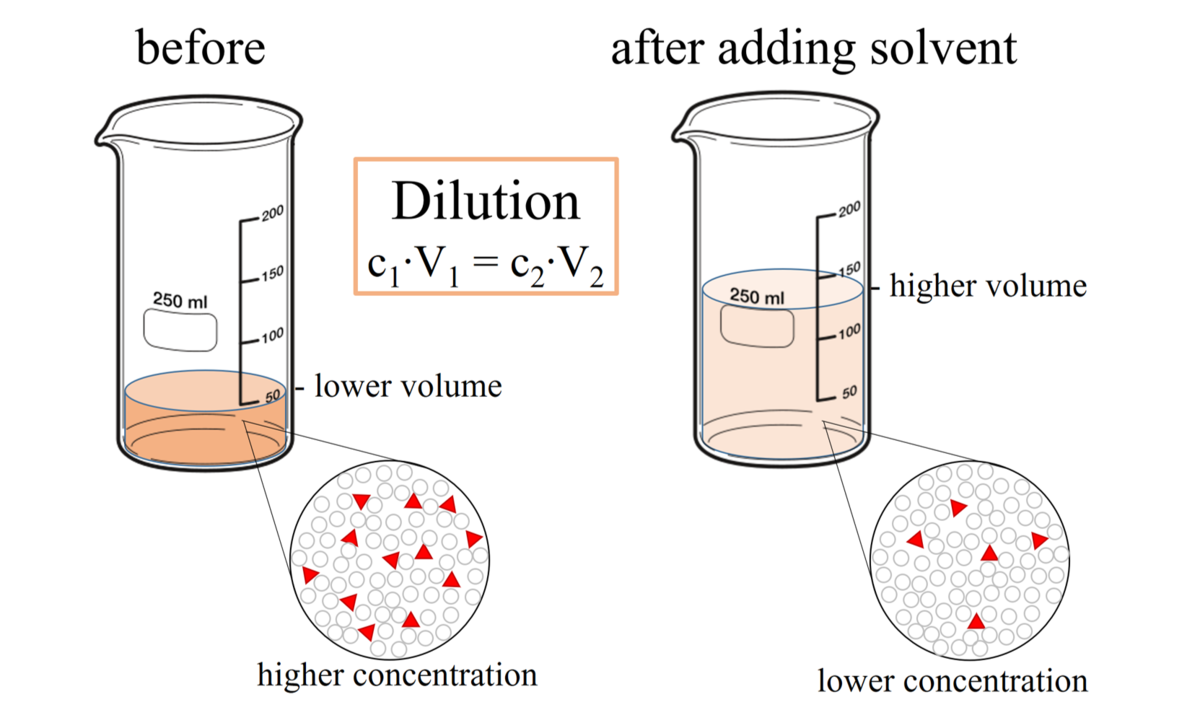
Solution Dilutions. D ilution In a dilution water is added. volume increases. concentration decreases. Copyright © 2009 by Pearson Education, Inc. - ppt download
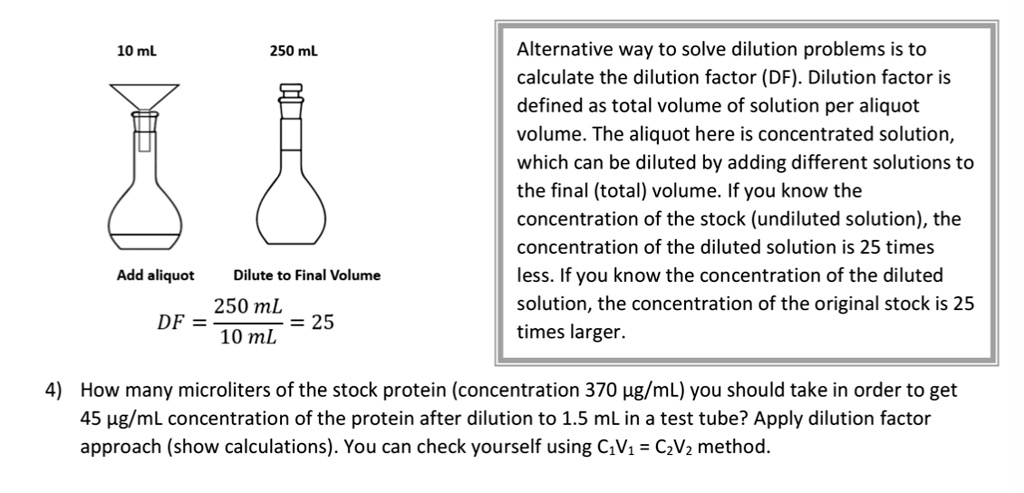
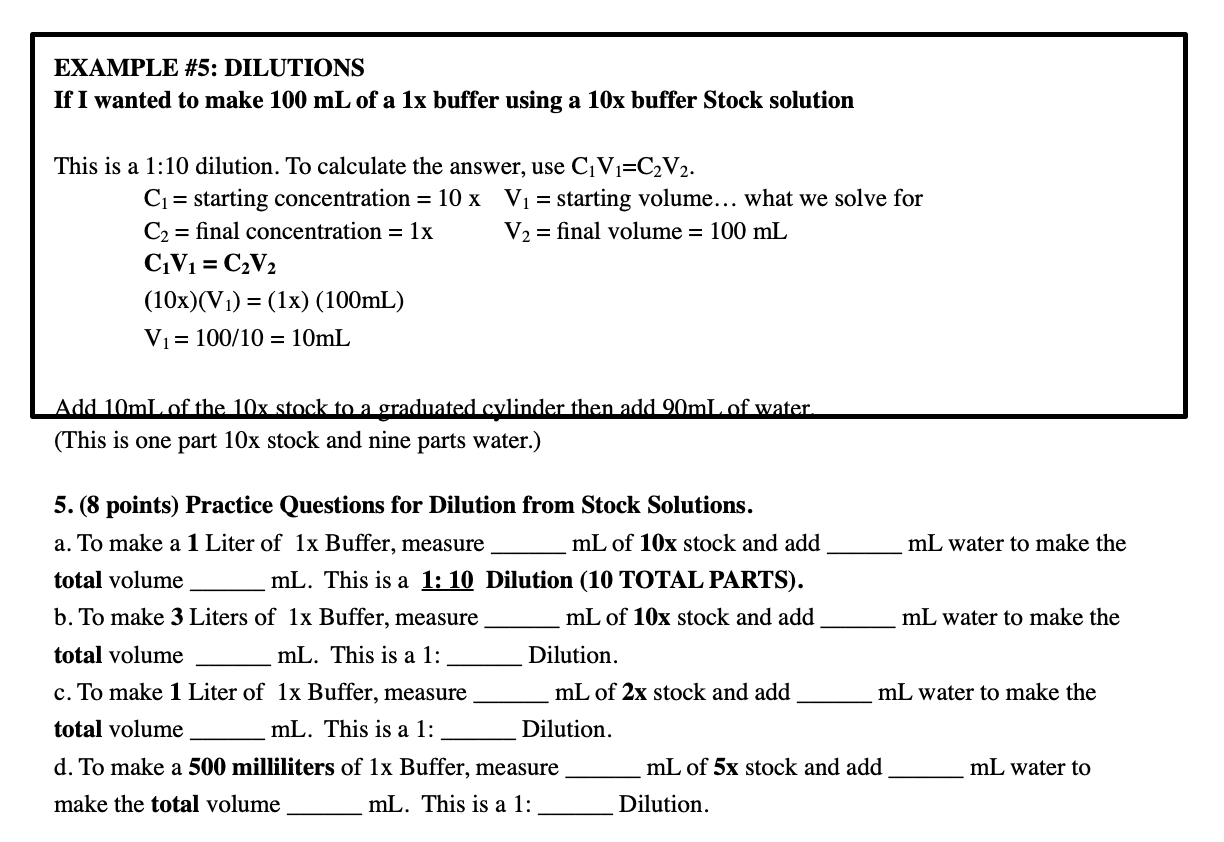
SOLVED: Alternative way to solve dilution problems is to calculate the dilution factor (DF): Dilution factor is defined as total volume of solution per aliquot volume. The aliquot here is concentrated solution,

Dilution Equation & Examples | How to Calculate Dilution Factors - Video & Lesson Transcript | Study.com

1 Chapter 10 Acids and Bases 10.10Dilutions. 2 Dilution Diluting a solution Is the addition of water. Decreases concentration. ConcentratedDilutedSolution. - ppt download