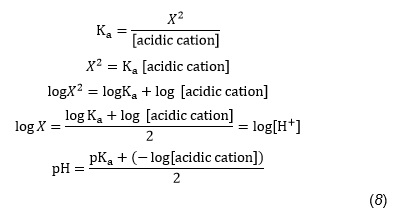
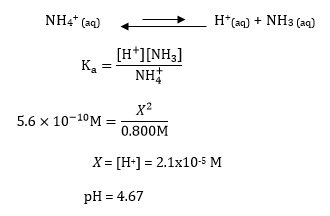
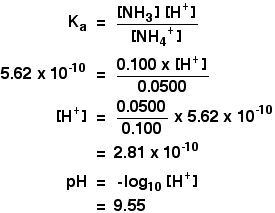pH of when 50mL of 0.10 M ammonia solution is treated with 50 mL of 0.05 M HCI solution :- ` - YouTube

Caculate the pH of a 0.10M ammonia solution. Calculate the pH after 50.0 ml of this solution is treated with 25.0 ml of 0.10M HCl . The dissociation constant of ammonia, Kb = 1.77 × 10^-5 .
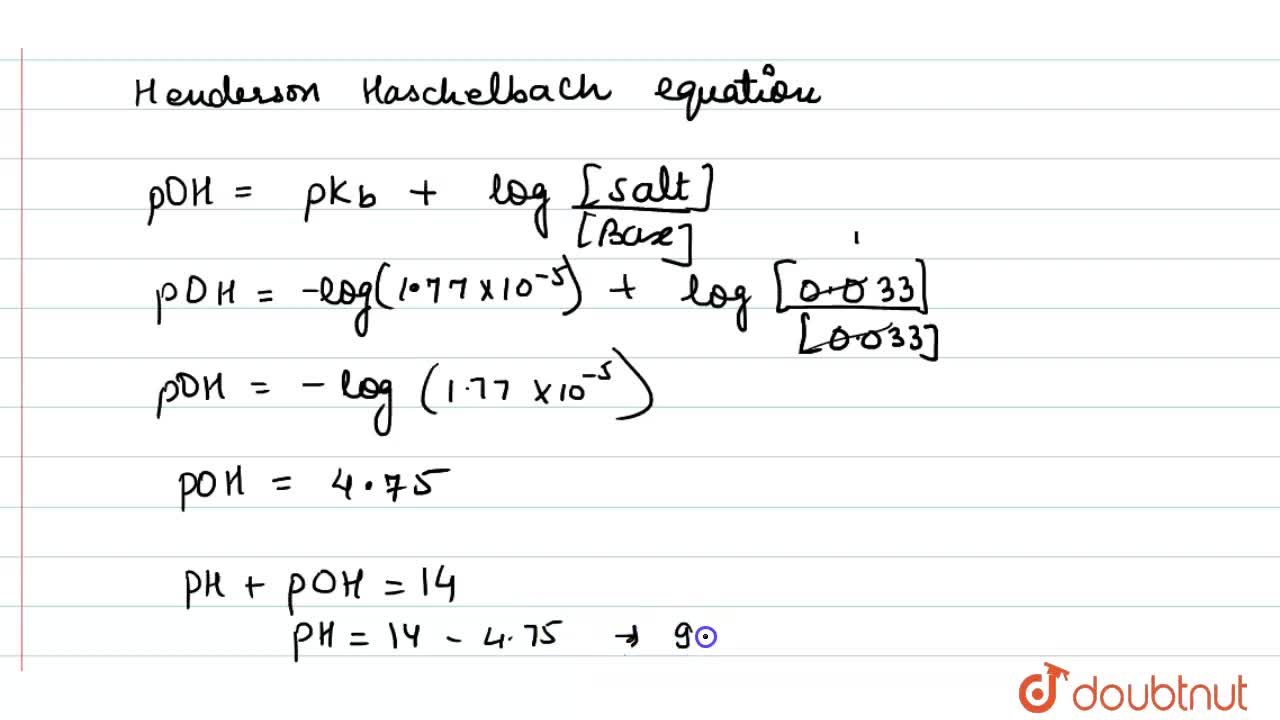
Calculate the pH of 0.033 M ammonia solution if 0.033 M NH(4)Cl is introduced in this solution at the same temperature (K(b) for NH(3)=1.77xx10^(-5))
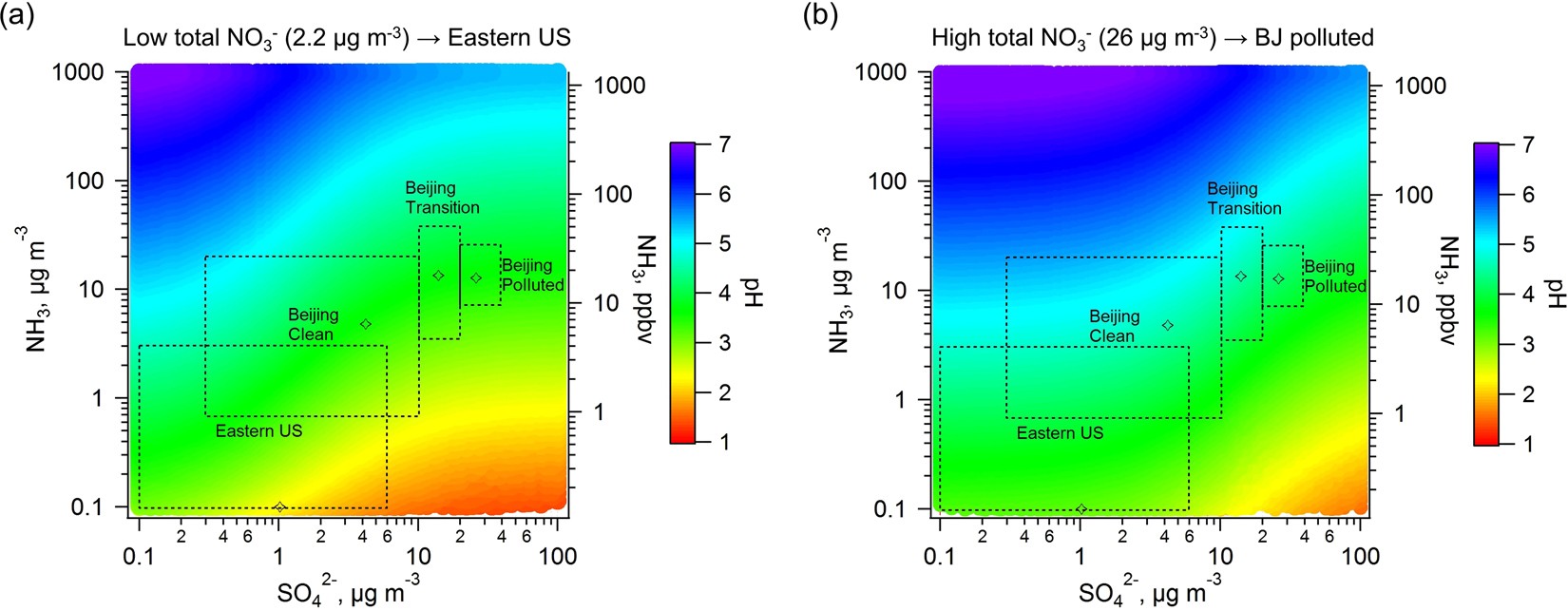
High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production | Scientific Reports

In the titration of 50.0 mL of 0.10 M ammonia (K_b = 1.8 times 10^{-5}), calculate the pH: 1 ) Before titration begins 2 ) After addition of 20.0 mL of 0.10 M hydrochloric acid 3 ) After addition | Homework.Study.com
![The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ]. The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].](https://dwes9vv9u0550.cloudfront.net/images/4298277/0914b99c-8837-49a9-86f7-3cbcdb1ec4a6.jpg)
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].