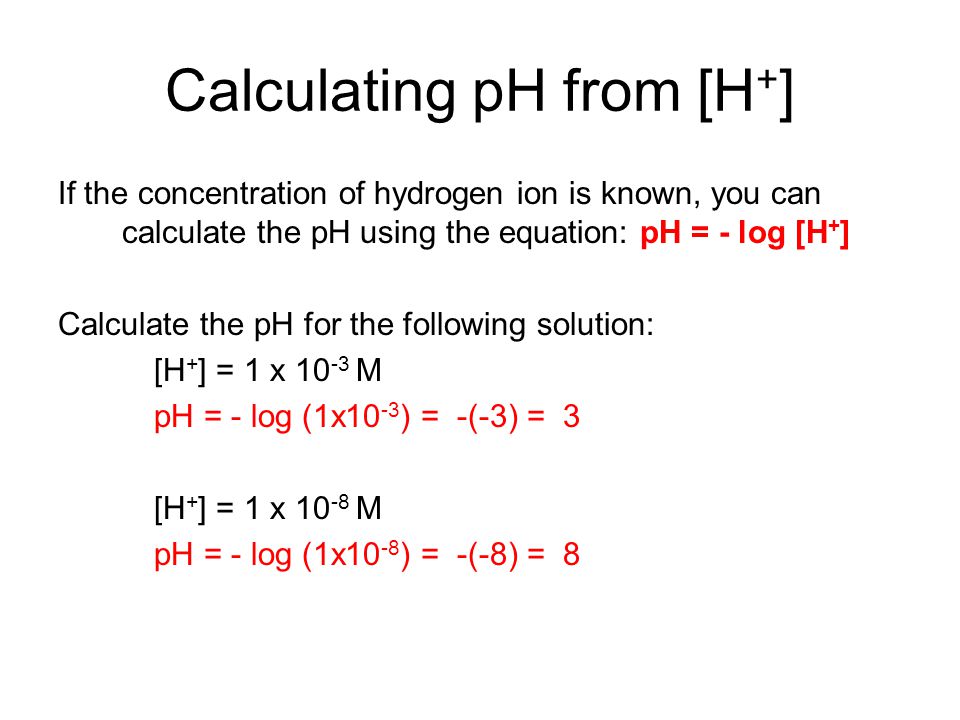
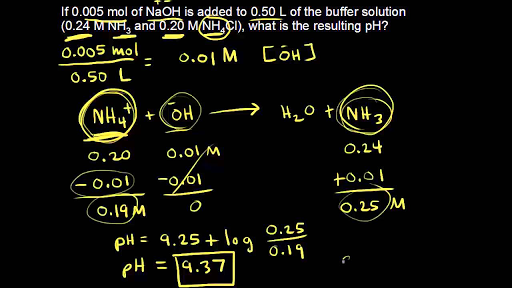
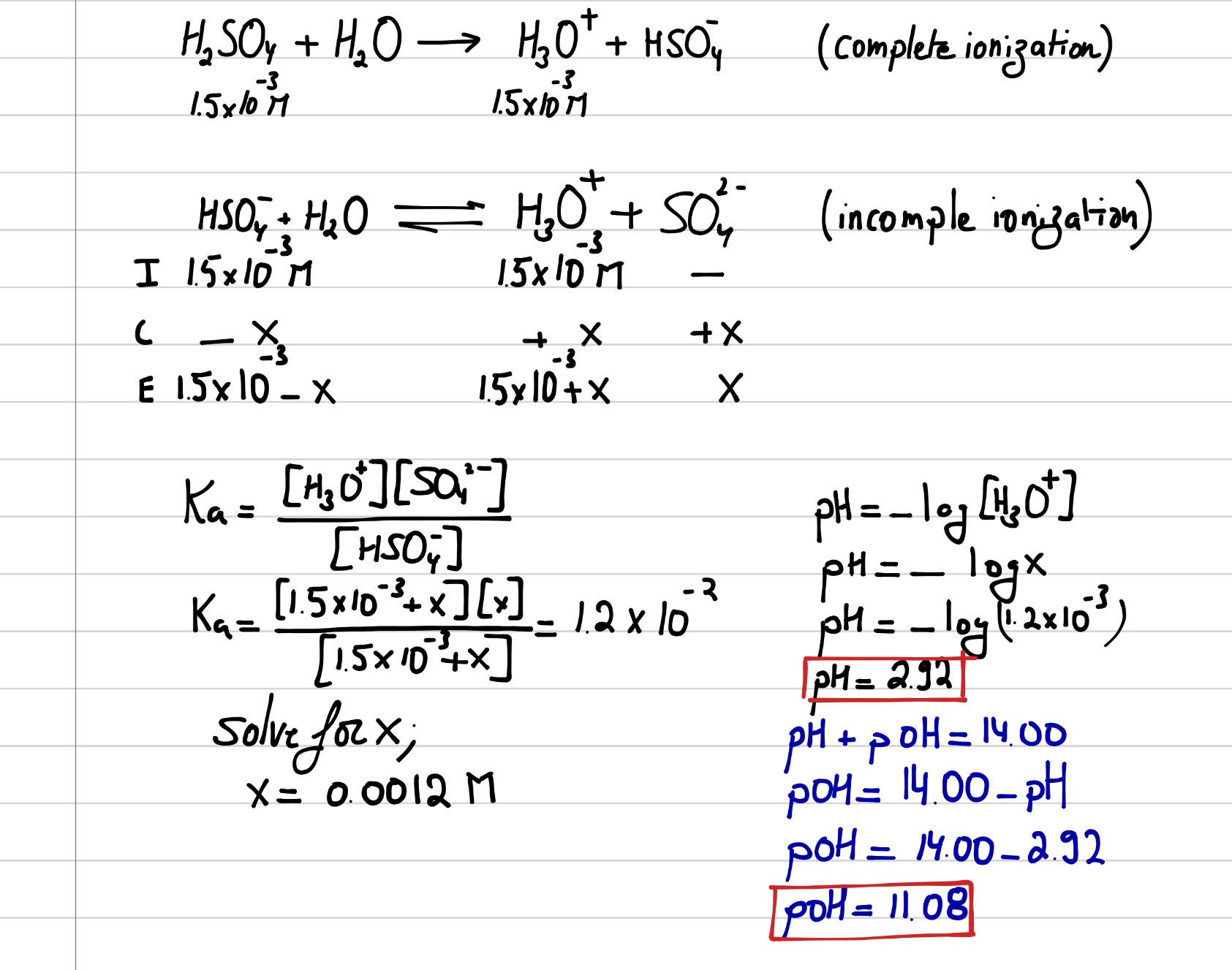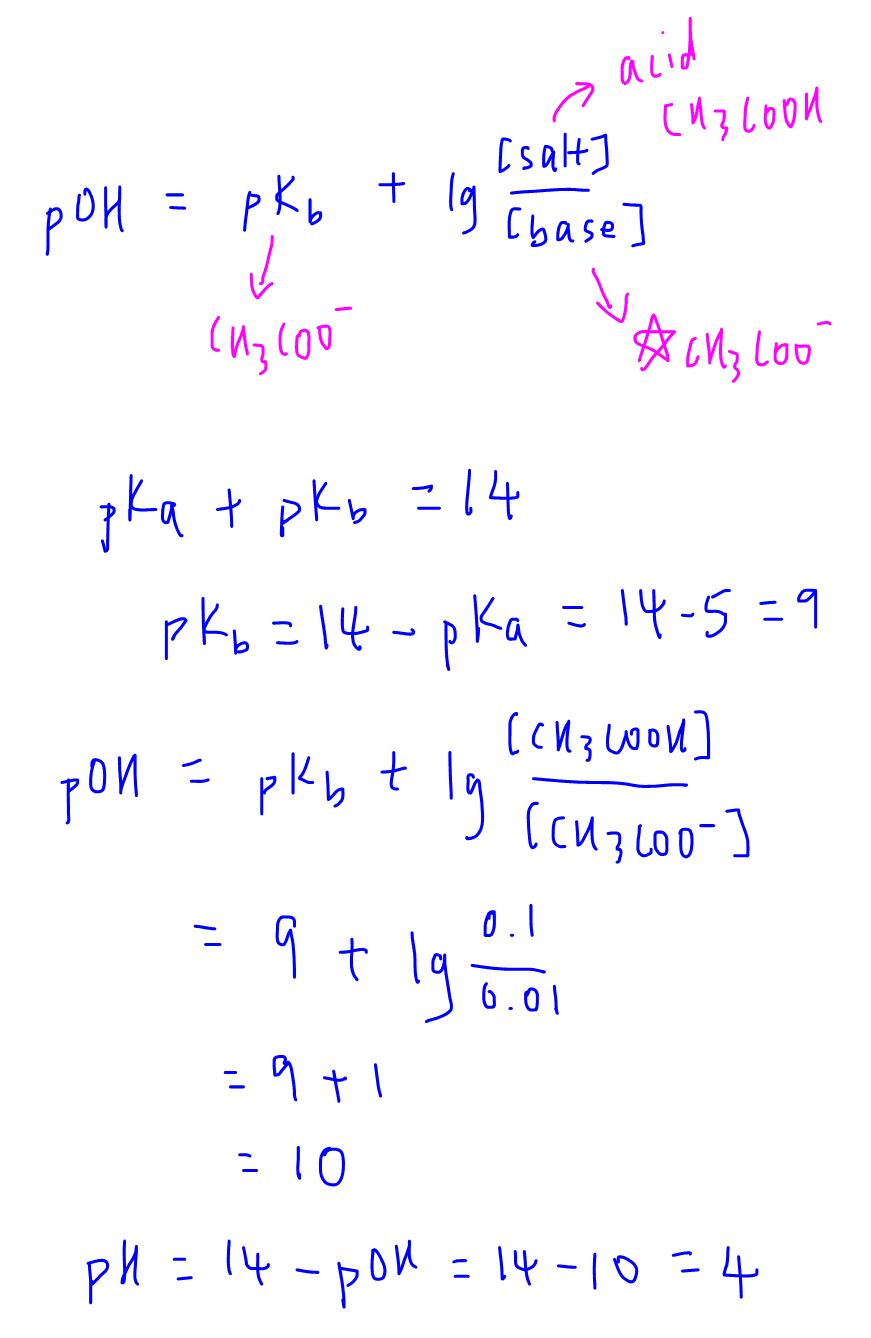25. Calculate the pH of a solution obtaining by mixing 200ml of. 1M CH3COOH (Ka=10 5) and 50ml of. 2M Ca(OH)2
Calculate the pH of the following solutions: - Sarthaks eConnect | Largest Online Education Community

SOLVED:Calculate the pH of the solution that results from each mixture. a. 150.0 mL of 0.25 M HF with 225.0 mL of 0.30 M NaF b. 175.0 mL of 0.10 M C2
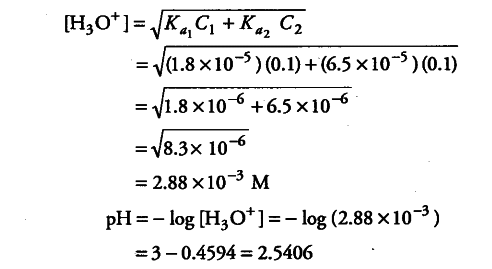
Calculate the pH of a solution containing 0.1 M acetic acid and 0.1 M benzoic acid - CBSE Class 11 Biology - Learn CBSE Forum
![Calculate pH of the solution which is 10^(-1)M in HCl & 10^(-3)M in CH(3)COOH[K(a)=2xx10^(-5)].Also calculate [H^(+)] form CH(3)COOH. Calculate pH of the solution which is 10^(-1)M in HCl & 10^(-3)M in CH(3)COOH[K(a)=2xx10^(-5)].Also calculate [H^(+)] form CH(3)COOH.](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/33101699_web.png)
Calculate pH of the solution which is 10^(-1)M in HCl & 10^(-3)M in CH(3)COOH[K(a)=2xx10^(-5)].Also calculate [H^(+)] form CH(3)COOH.
![The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ]. The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].](https://dwes9vv9u0550.cloudfront.net/images/4298277/0914b99c-8837-49a9-86f7-3cbcdb1ec4a6.jpg)
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].
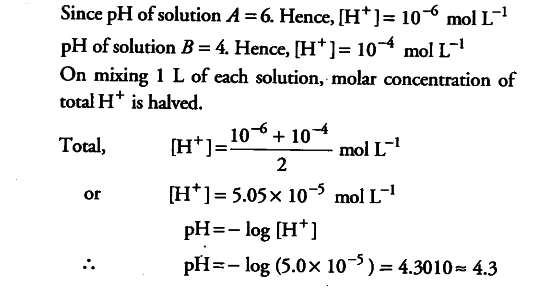
Calculate the pH of a solution formed by mixing equal volumes of two solutions, - CBSE Class 11 Chemistry - Learn CBSE Forum

Calculate the ph of a solution formed by mixing equal volumes of two solutions A and B of a strong acids having ph=6" and "ph=4 respectively.