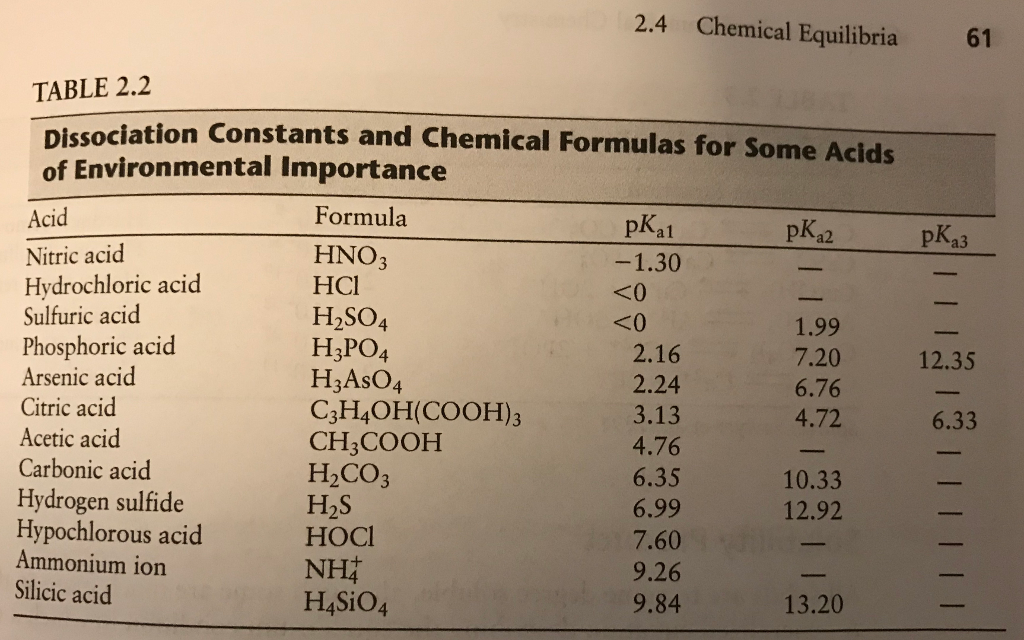
Which of the species from the equilibrium below are conjugate acid-base pairs? NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com

Acid-base reaction with H2S liberation versus time. H2S detection via... | Download Scientific Diagram
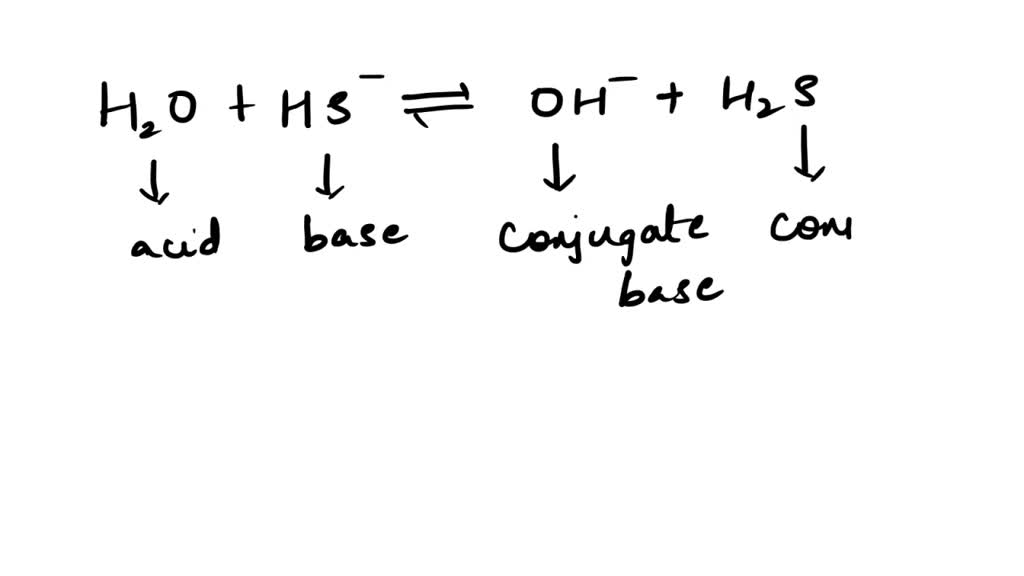
SOLVED: Be sure to answer all parts. In the following equation, identify the acids, bases, and conjugate pairs. H2O + HS− ⇌ OH− + H2S (a) What is the acid? H2O HS−

Experts please tell whether H2S is lewis acid or lewis base and bronsted acid or bronsted base - Chemistry - Hydrogen - 16684677 | Meritnation.com

Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com
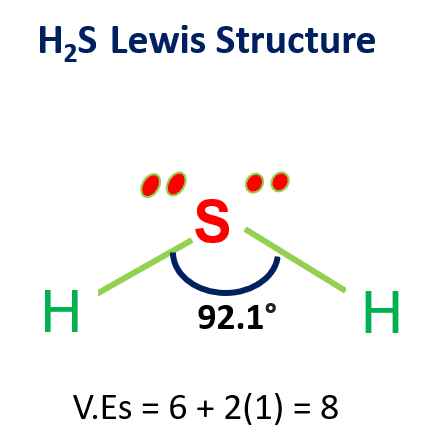
SOLVED: In aqueous solution, HS- is the conjugate acid of and the conjugate base of . Group of answer choices H2S, S2- H3O+, S2- S2-, H2S S2-, H3O+

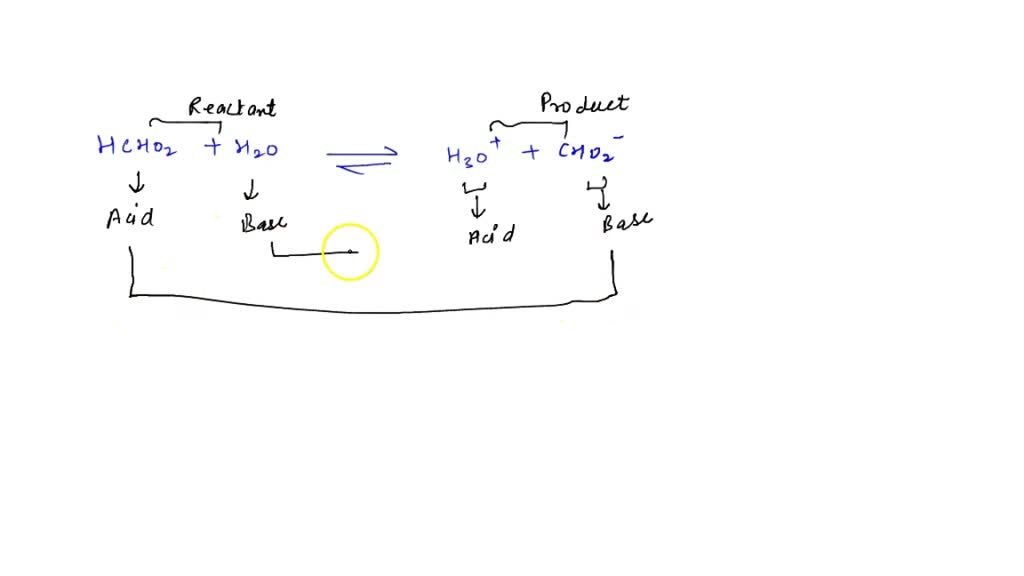
SOLVED: Write an equation that shows the reaction of hydrogen sulfide, HS– with hydroxide ion, OH–. Label the acid, the base, the conjugate acid, and the conjugate base.


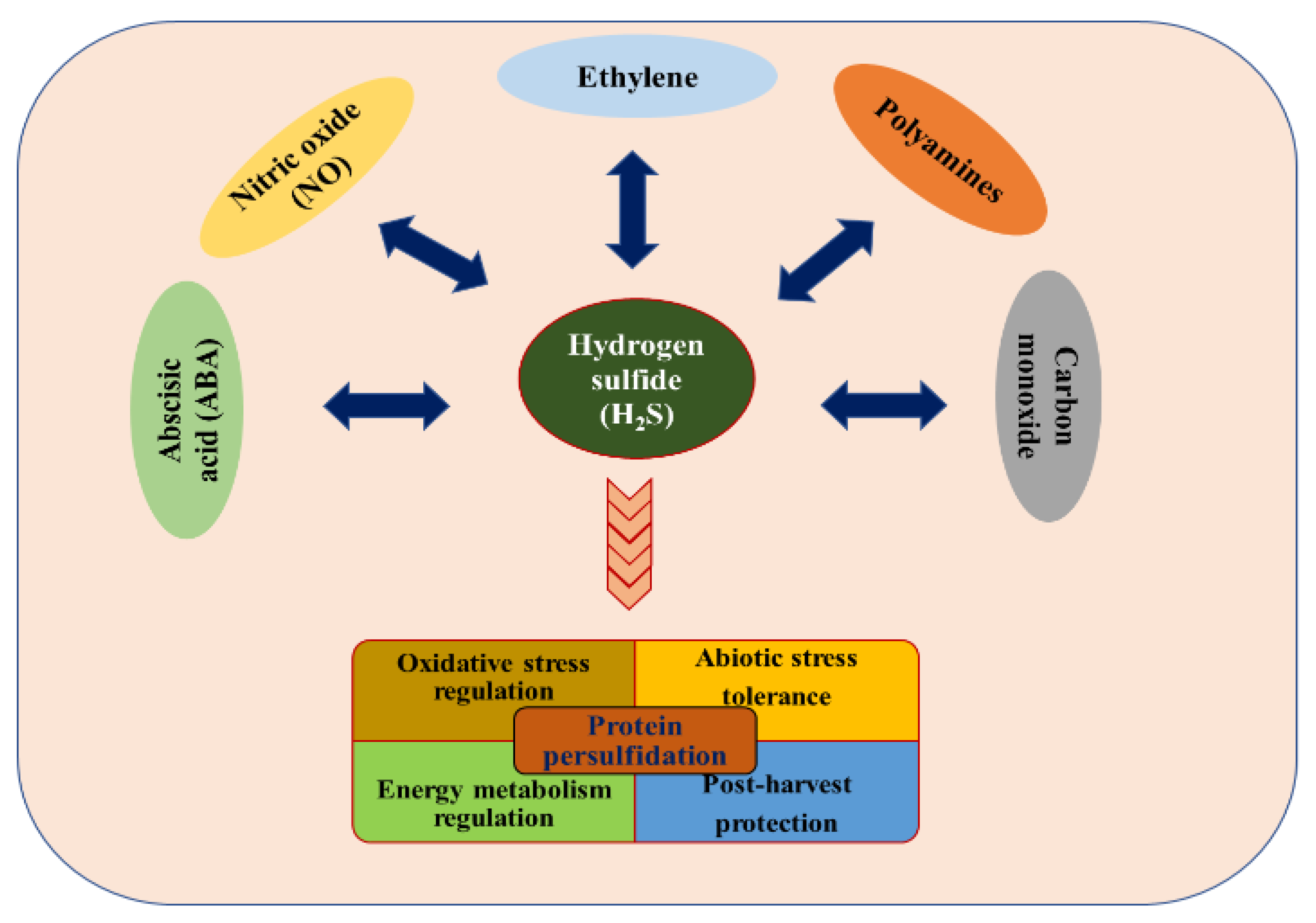




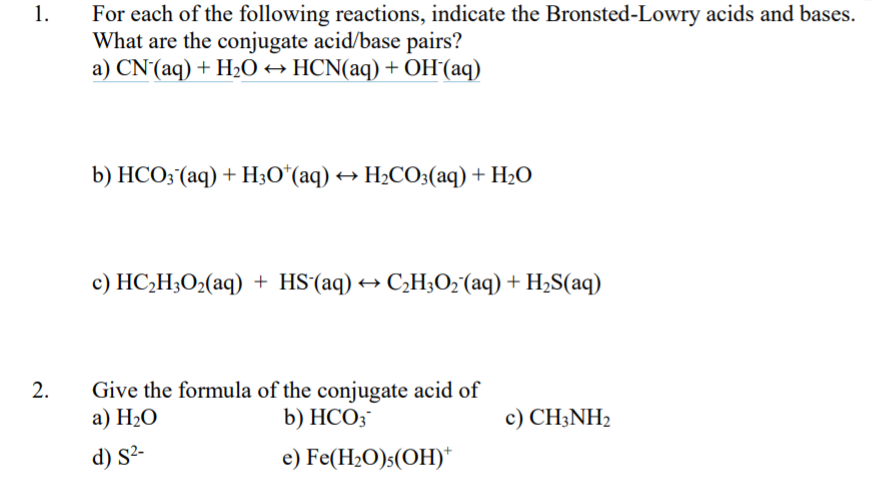
.jpg)