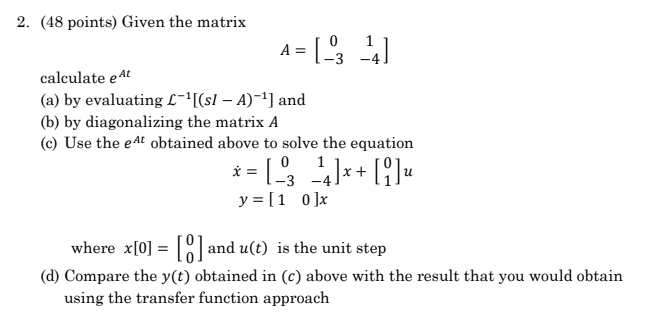
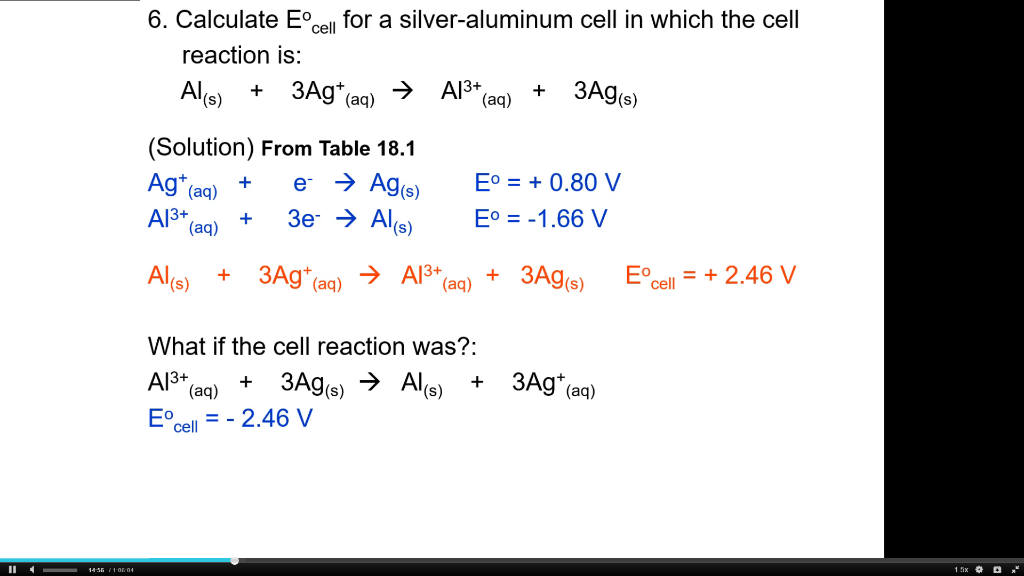
Write the cell reaction and calculate E^o cell of the following electrochemical cell: (s)Al| (1M)(aq.)Al^3 + || (1M) (aq.)Zn^2 + | (s)Zn E^oAl = - 1.66 V E^oZn = - 0.76 V

probability theory - How to calculate $\mathbb{E}\left(T_{n}\right)$ and $\mathbb{E}\left(T^2_{n}\right)$? - Mathematics Stack Exchange

Half-cell potentials Electrochemical Series using E cell predicting reaction feasibility A level GCE AS A2 chemistry revision notes KS5

ELECTROCHEMISTRY Chap 20. Electrochemistry Sample Exercise 20.6 Calculating E° cell from E° red Using the standard reduction potentials listed in Table. - ppt download
Calculate E°cell for the following reaction at 298 K: - Sarthaks eConnect | Largest Online Education Community
![Given that standard potential for the following half - cell reaction at 298 K, Cu^(+)(aq)+e^(-)rarr Cu(s), E^(@)=0.52V Cu^(2+)(aq)+e^(-)rarrCu^(+)(aq), E^(@)=0.16V Calculate the DeltaG^(@)(kJ) for the eaction, [2Cu^(+)(aq)rarr Cu(s)+Cu^(2+)] Given that standard potential for the following half - cell reaction at 298 K, Cu^(+)(aq)+e^(-)rarr Cu(s), E^(@)=0.52V Cu^(2+)(aq)+e^(-)rarrCu^(+)(aq), E^(@)=0.16V Calculate the DeltaG^(@)(kJ) for the eaction, [2Cu^(+)(aq)rarr Cu(s)+Cu^(2+)]](https://d10lpgp6xz60nq.cloudfront.net/question-thumbnail/en_327414305.png)









![probability theory - How to calculate E[Xi Xj]? - Mathematics Stack Exchange probability theory - How to calculate E[Xi Xj]? - Mathematics Stack Exchange](https://i.stack.imgur.com/ZroYQ.jpg)


![probability - How to calculate $E[X]$ of a Poisson random variable. - Mathematics Stack Exchange probability - How to calculate $E[X]$ of a Poisson random variable. - Mathematics Stack Exchange](https://i.stack.imgur.com/utxeE.png)