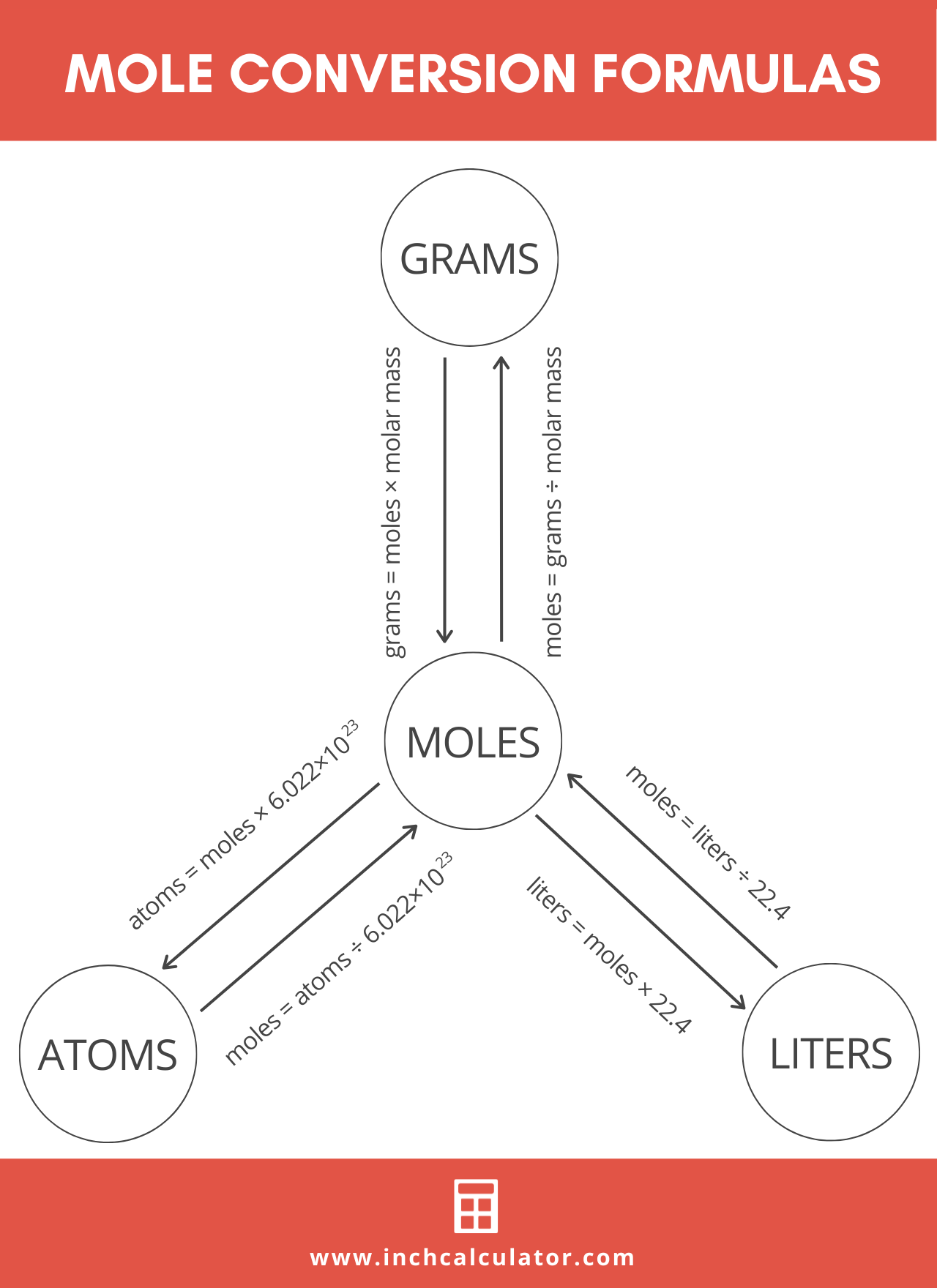
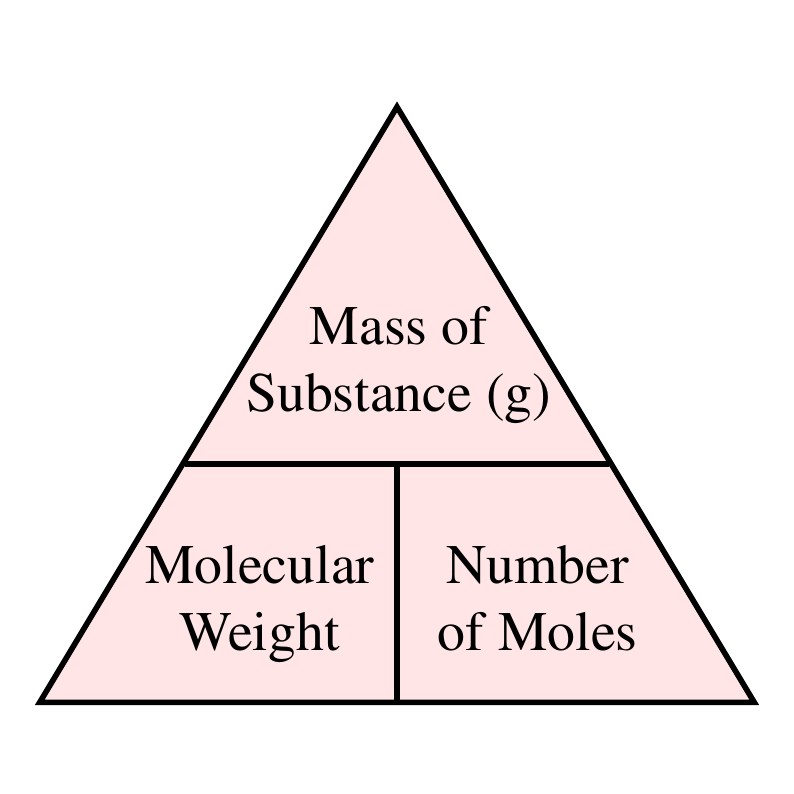
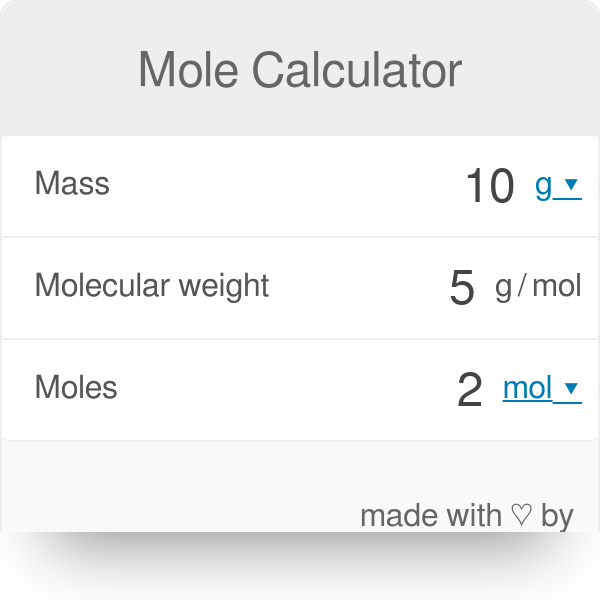
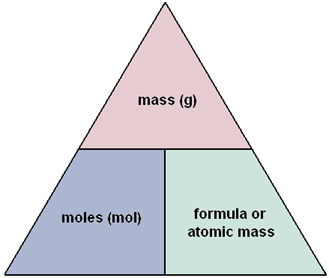
Calculate the number of moles for the following: 52 g of He (finding mole from mass) 12.044 × 10^ 23 number of He atoms (finding mole from number of particles)
Calculate the number of moles of hydrogen gas present in 500cm^3 of the gas taken at 300k and 760mm pressure. If this sample of hydrogen is found to have a mass equal
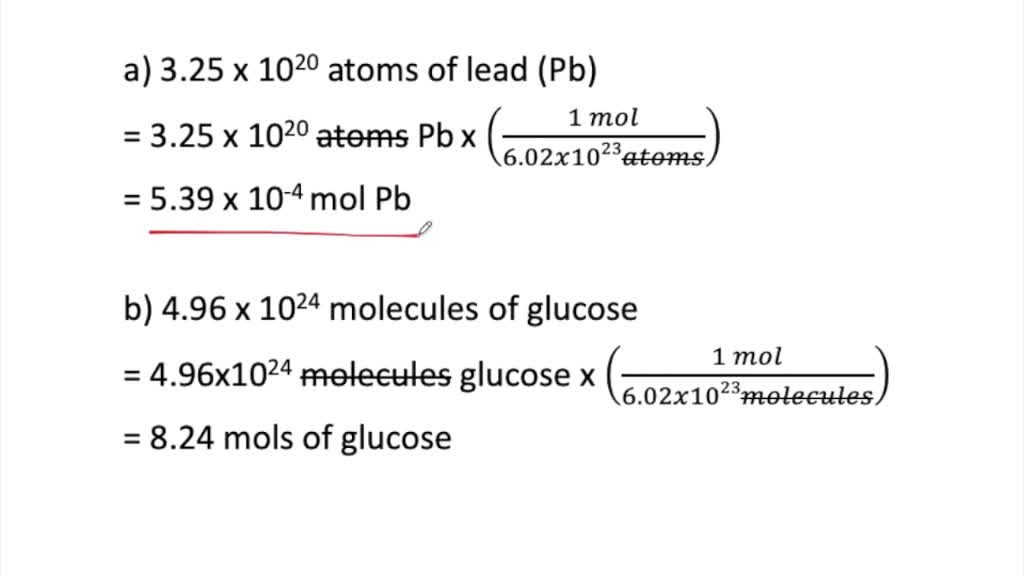
SOLVED:Determine the number of moles in each substance. a. 3.25 ×10^20 atoms of lead b. 4.96 ×10^24 molecules of glucose c. 1.56 ×10^23 formula units of sodium hydroxide d. 1.25 ×10^25 copper (II) ions