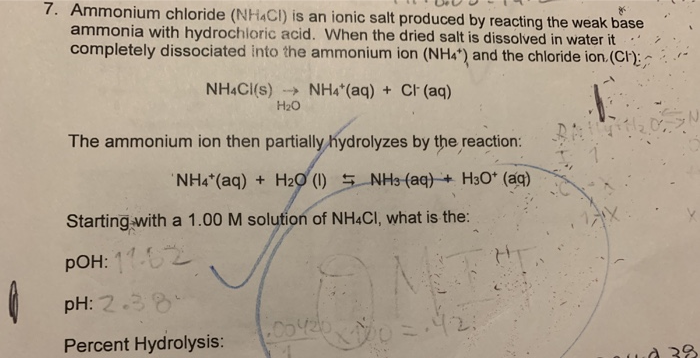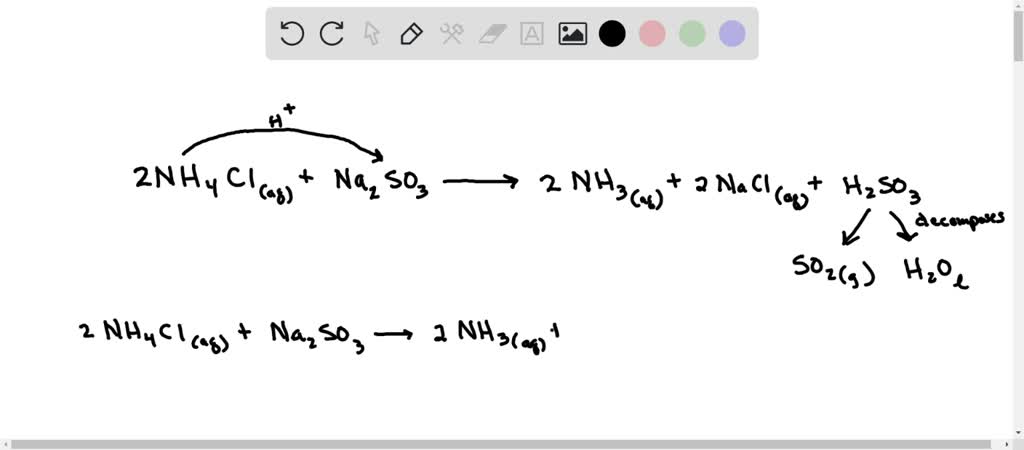
SOLVED: what's the acid base reaction for NH4Cl + Na2SO3 I keep putting in NH4^+ + SO3^2- –> NH3 + HSO3^- but it tells me its not an equilibrium

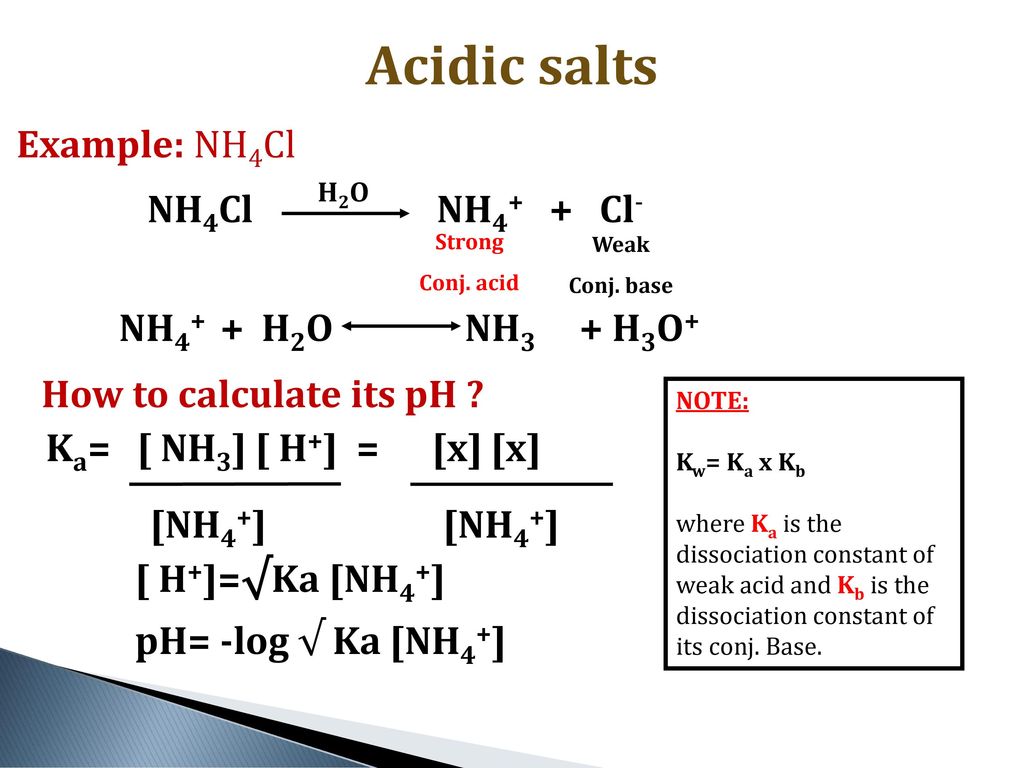
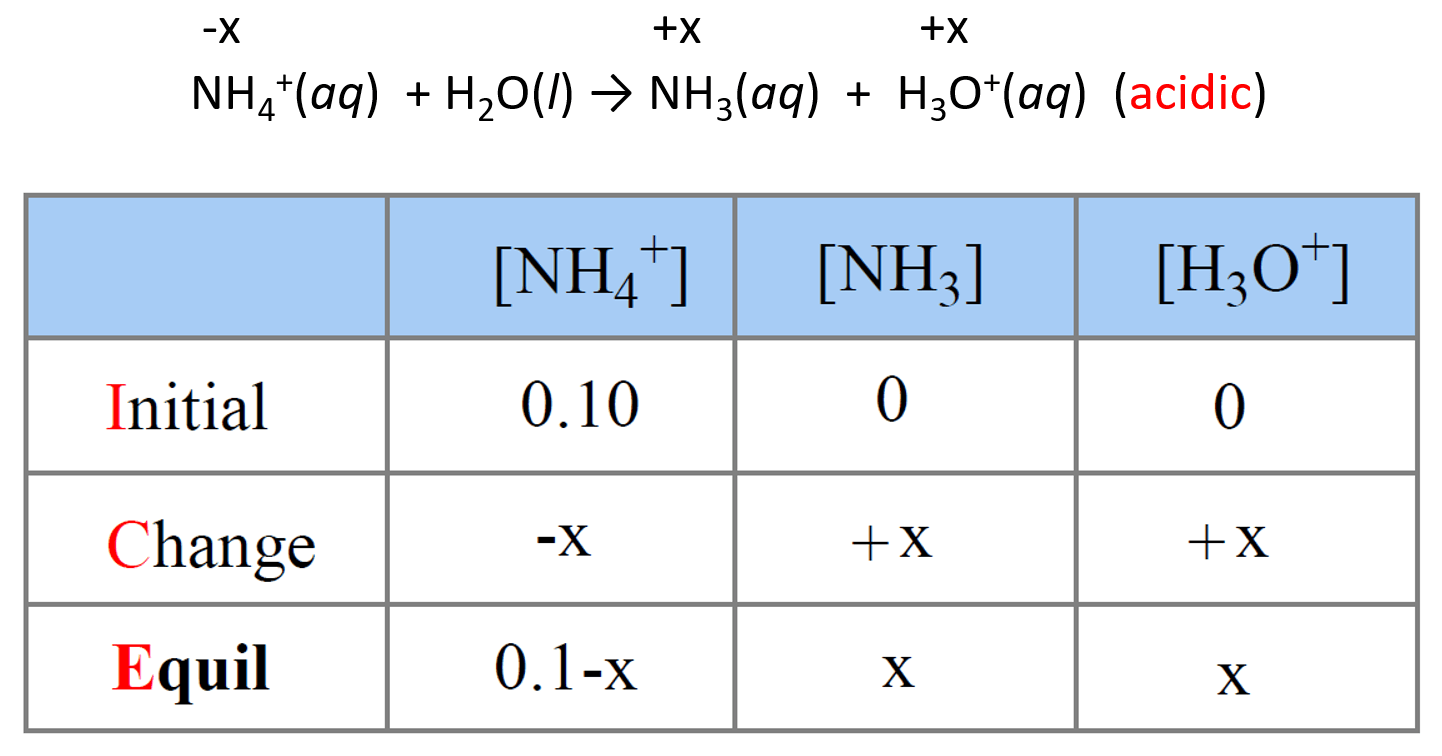
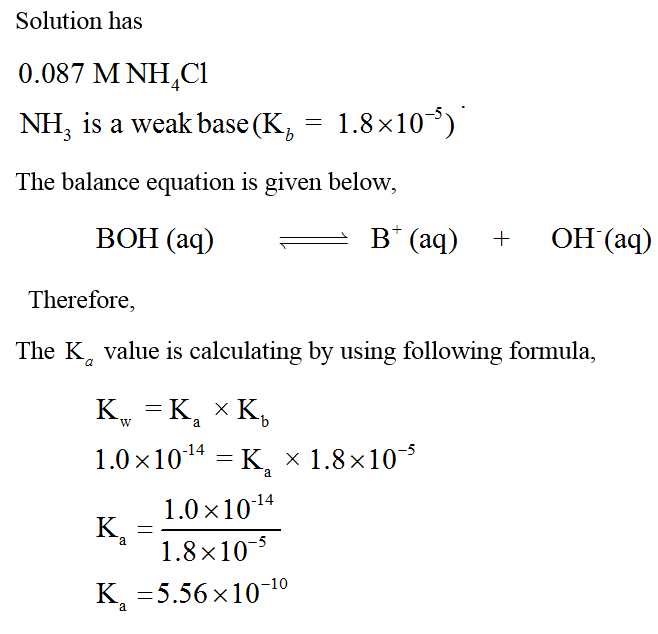
NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in

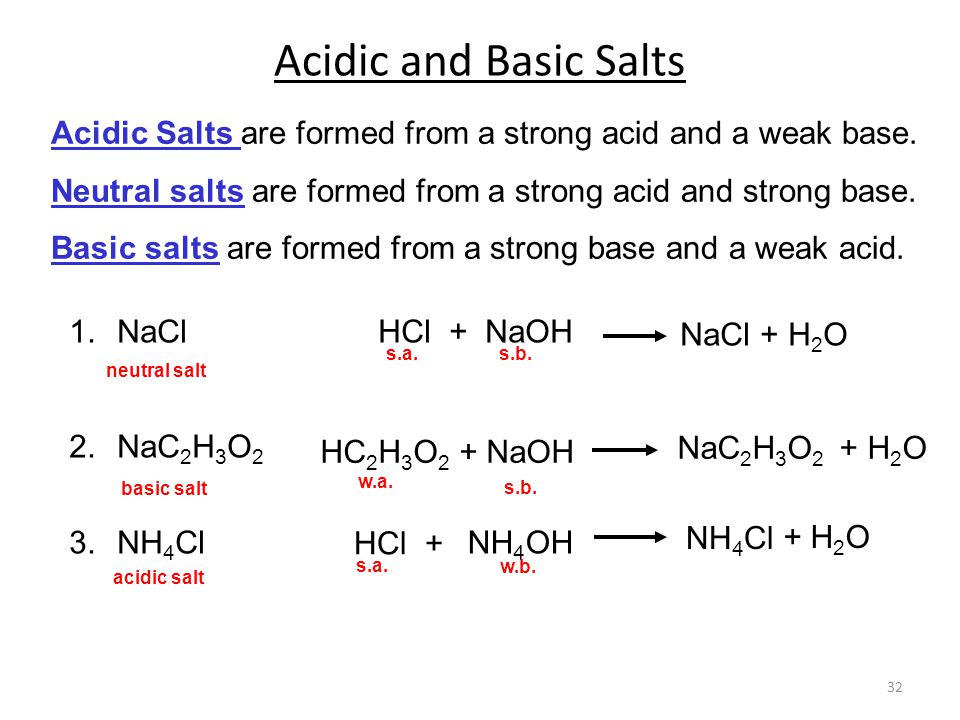
Why an aqueous solution of NH4Cl is acidic while that of HCOOK is basic - Chemistry - Ionic Equilibria - 16488273 | Meritnation.com

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in
CHEM 1332 (A.M. Guloy) CHEMICAL EQUILIBRIA--ACID/BASE Acid/base problems may fall into 4 categories: strong acid/base, weak acid
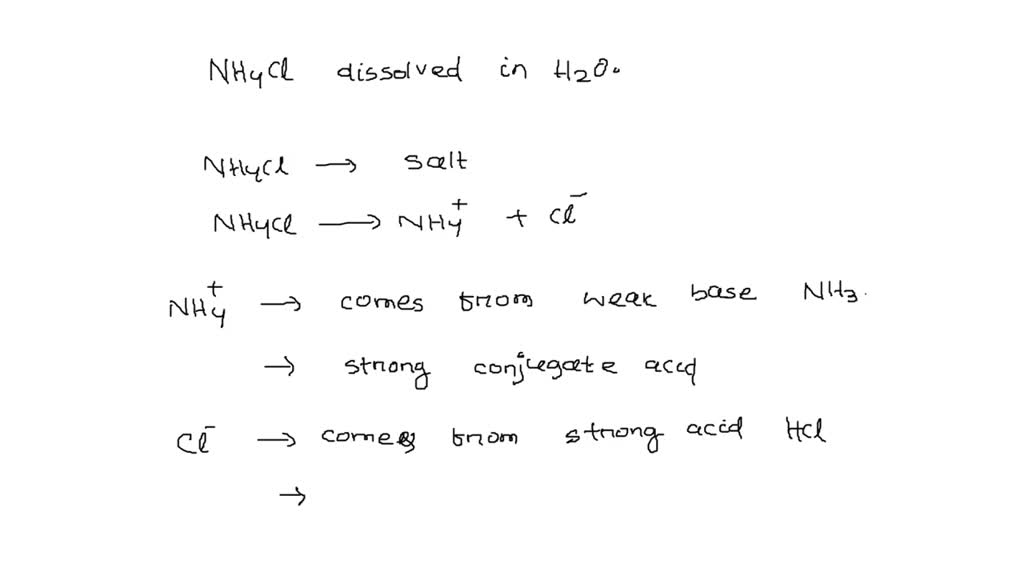
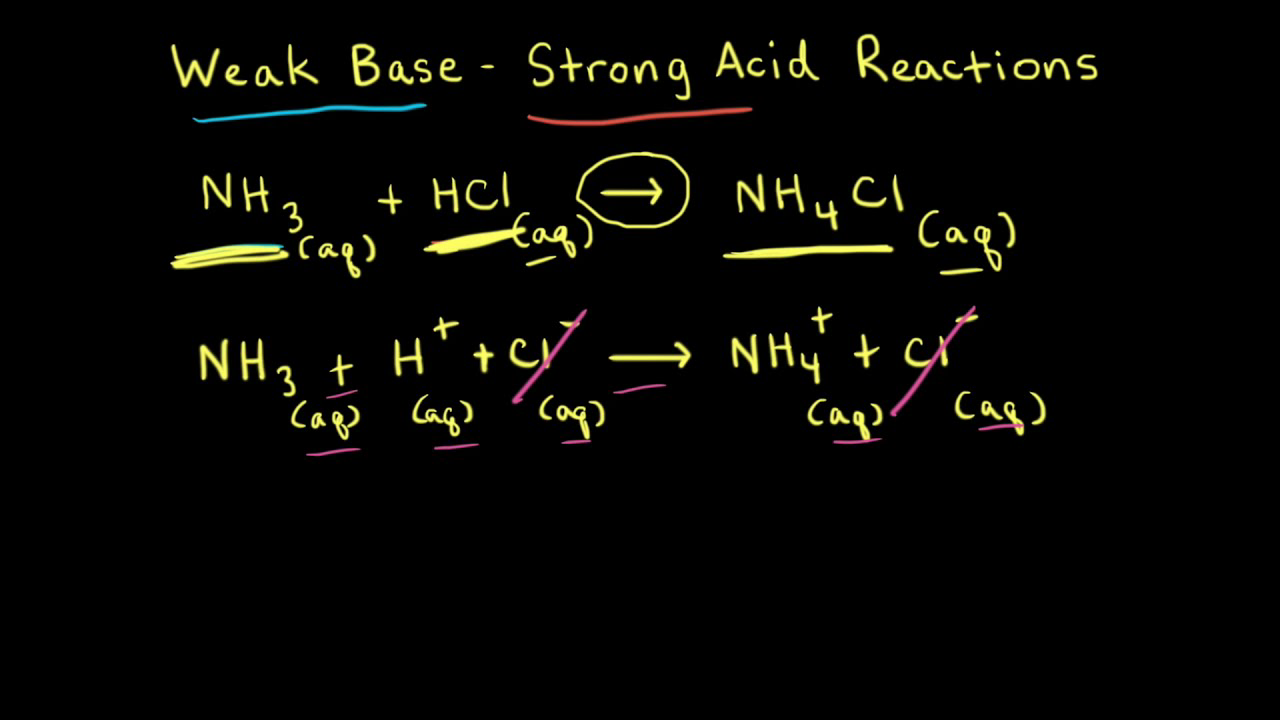
SOLVED: Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium chloride is dissolved in water. (Use H3O+ instead of H+.) + H2o(l) = + is the
How to predict whether an aqueous solution of the following salts will be acidic, basic, or neutral? Justify your answers: KCl, NaCN, NH4NO3, and NH4C2H3O2 NH4CN - Quora