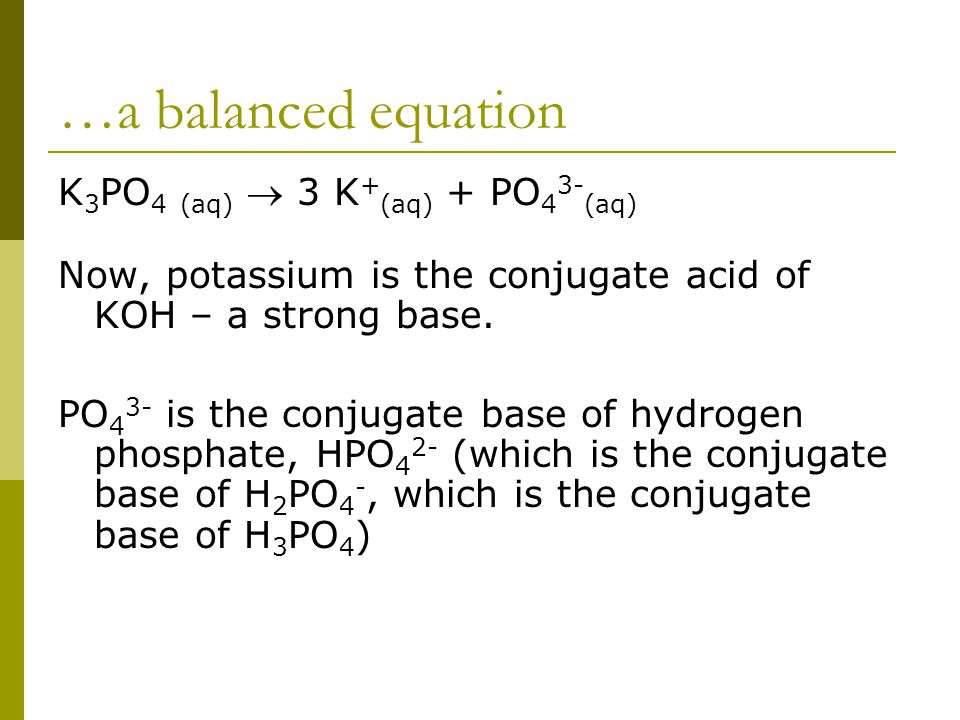
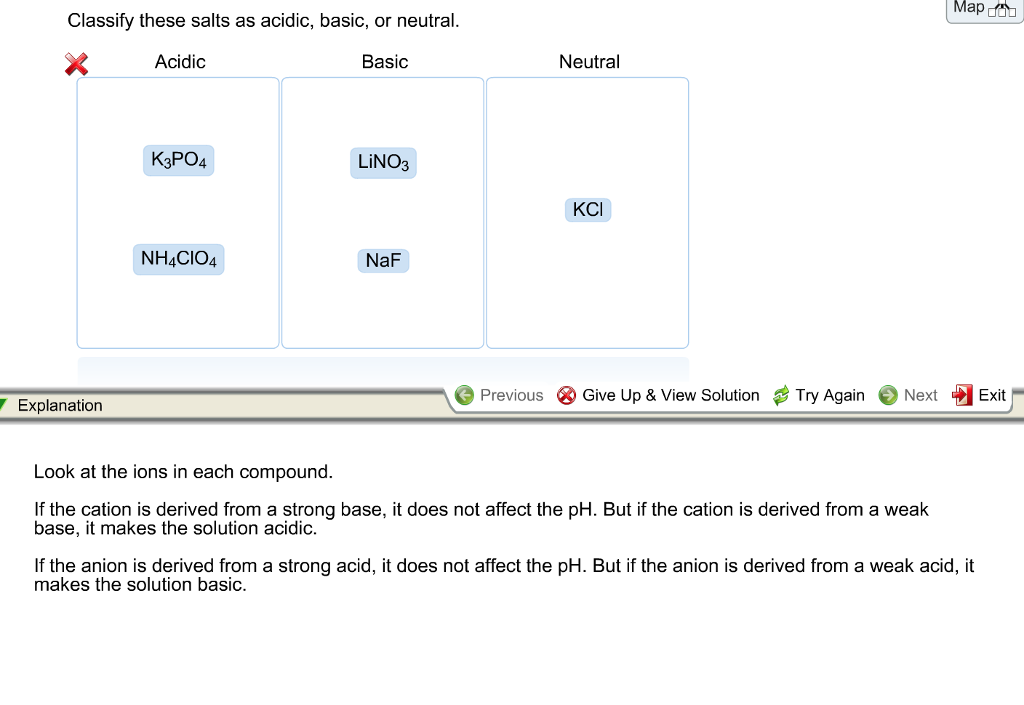
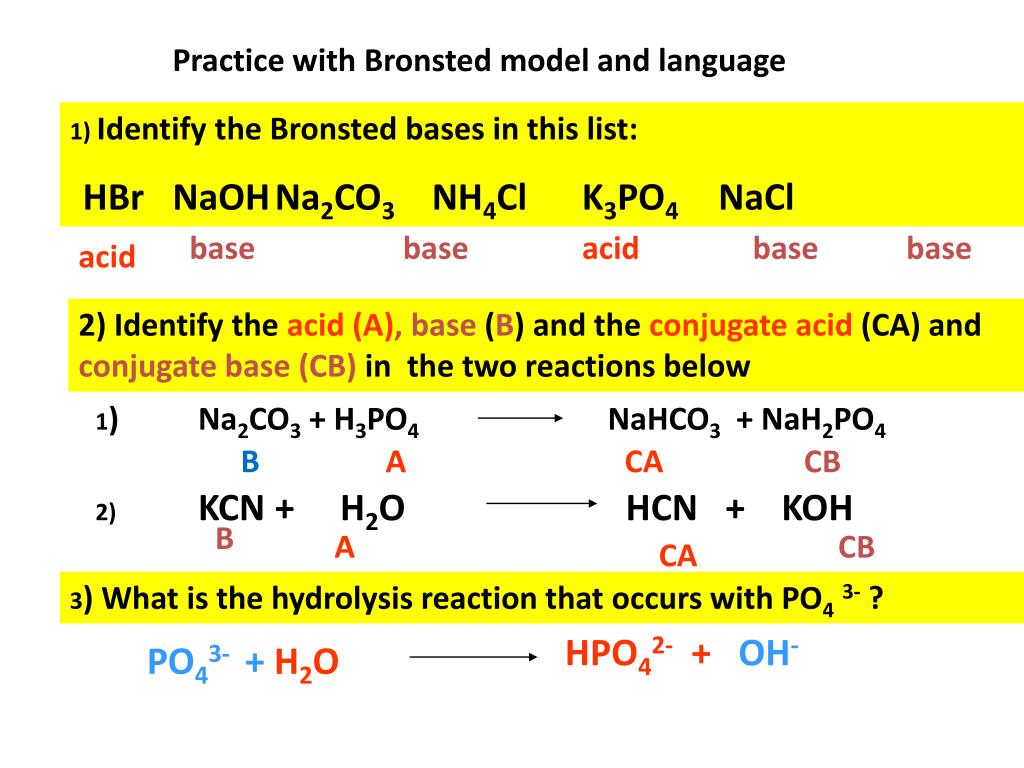
The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production - ScienceDirect
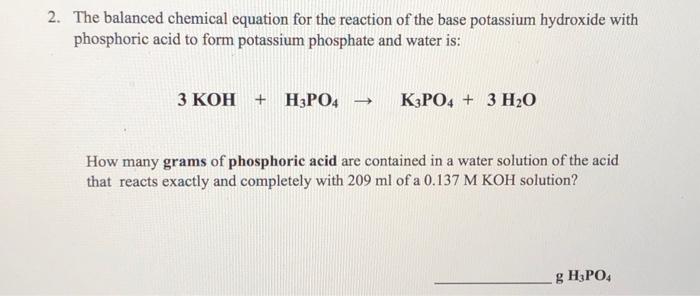
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams

Synthesis of HTLcs modified by K3PO4 for side chain alkylation of toluene with methanol - ScienceDirect

Air-Tolerant Direct Thiol Esterification with Carboxylic Acids Using Hydrosilane via Simple Inorganic Base Catalysis | The Journal of Organic Chemistry

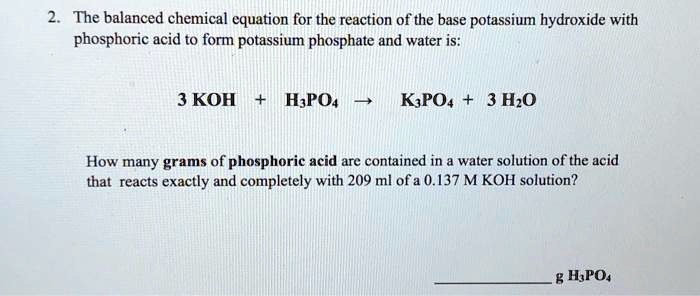
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams