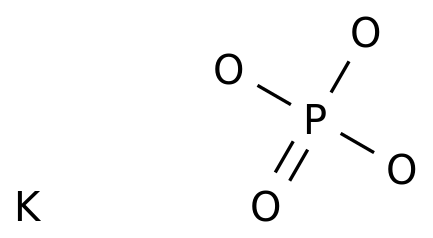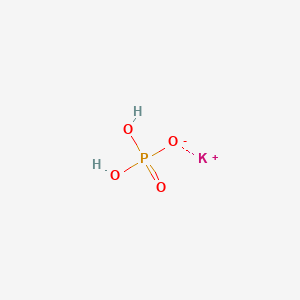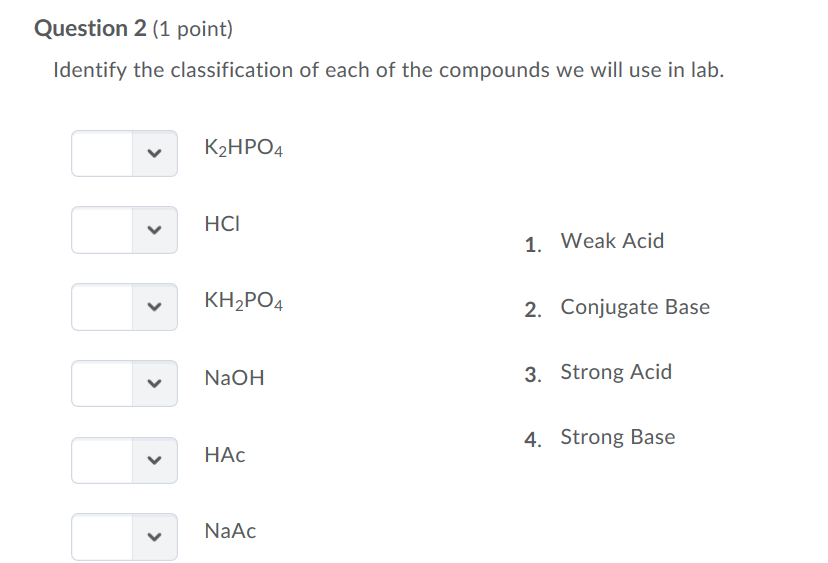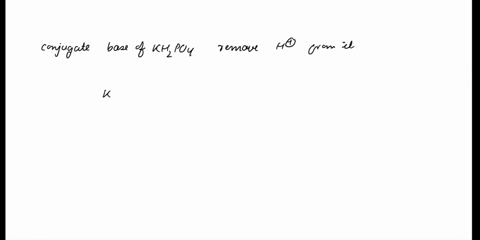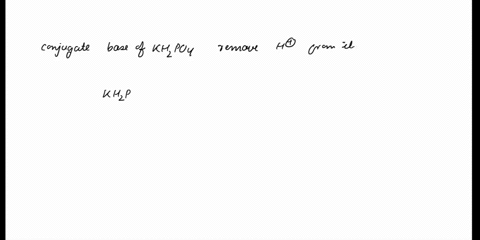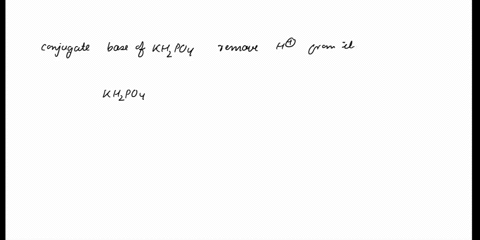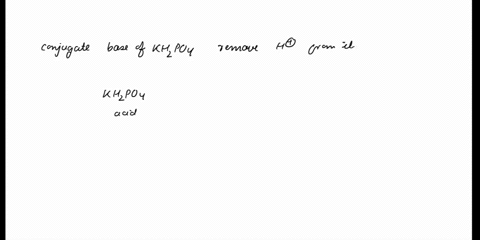
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?
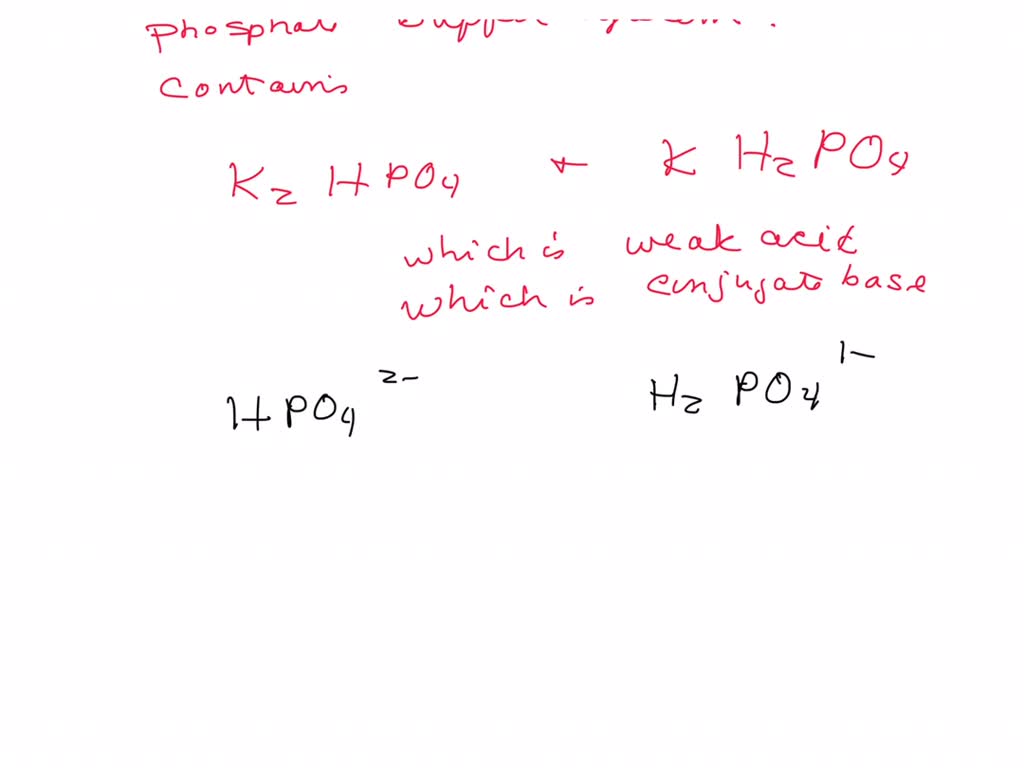
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?

Enhanced Second-Harmonic-Generation Response in a KH2PO4-Type Calcium Nitrate Carboxylate with Unusual Three-Dimensional Inorganic and Organic Connections | Inorganic Chemistry
![Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/KH2PO4%20500G.jpg)
Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs

A Process for removing toxic heavy metals to produce the high purity NH4H2PO4 and KH2PO4 from a crude phosphoric acid - ScienceDirect

If I want to make a potassium phosphate buffer solution, could I use KOH and H3PO4 instead of KH2PO4 and K2HPO4? | ResearchGate
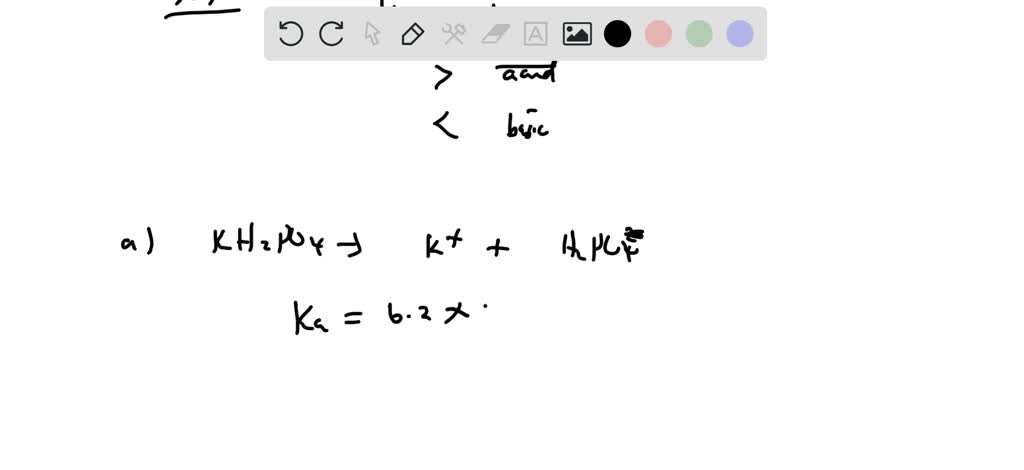
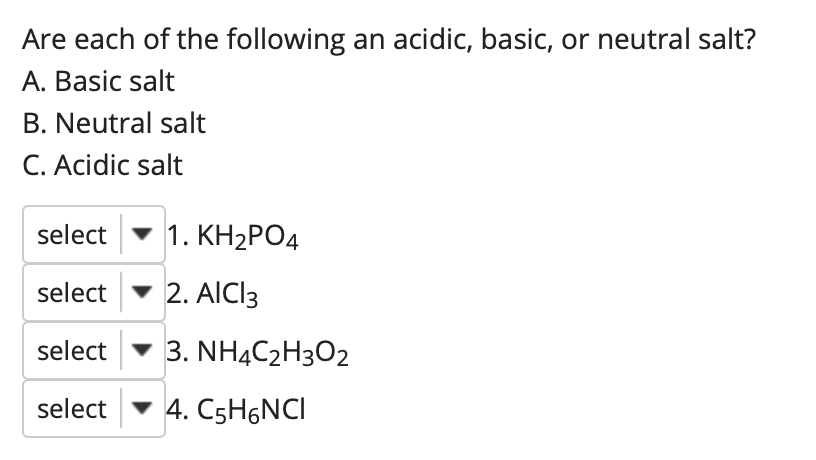
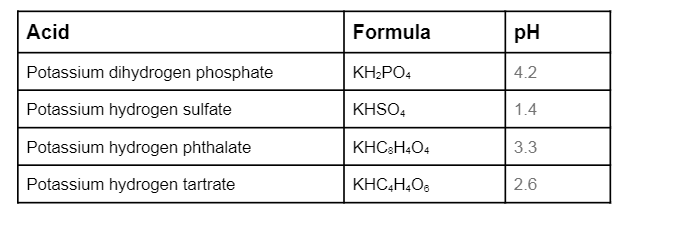
SOLVED:State whether each of the following solutions is acidic, basic, or neutral. (a) potassium dihydrogen phosphate (b) potassium hydrogen carbonate






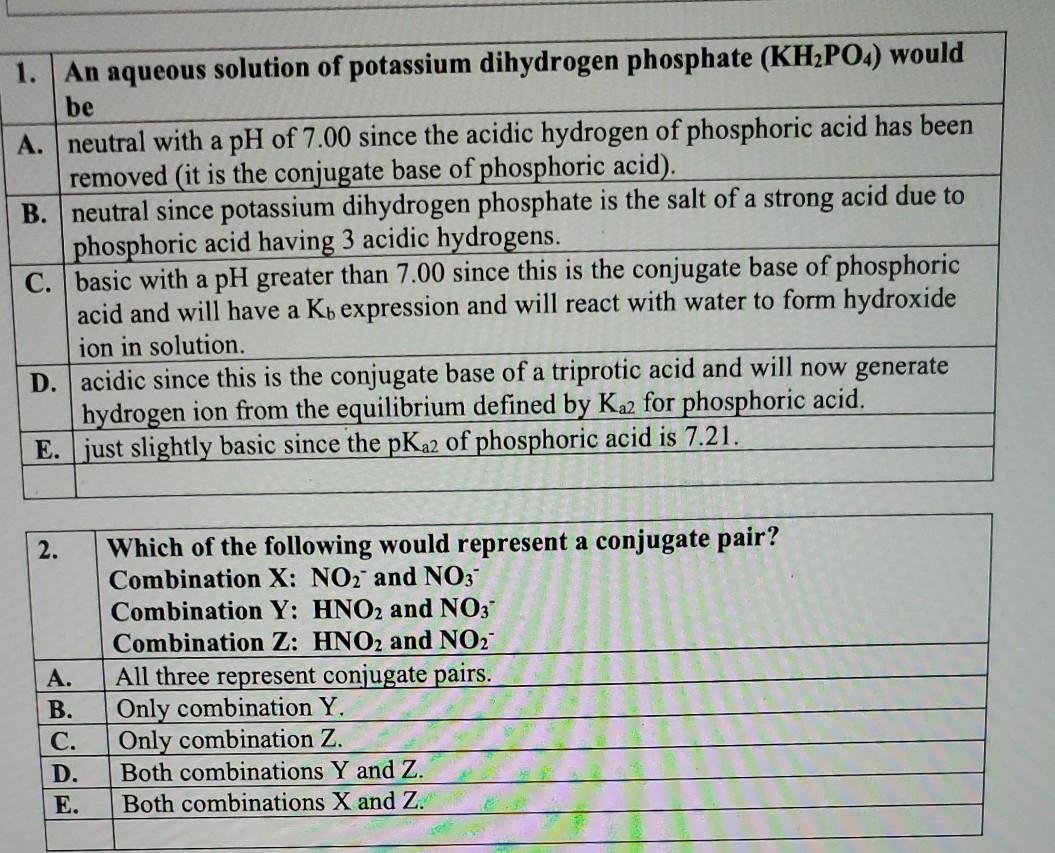
(358).jpg)