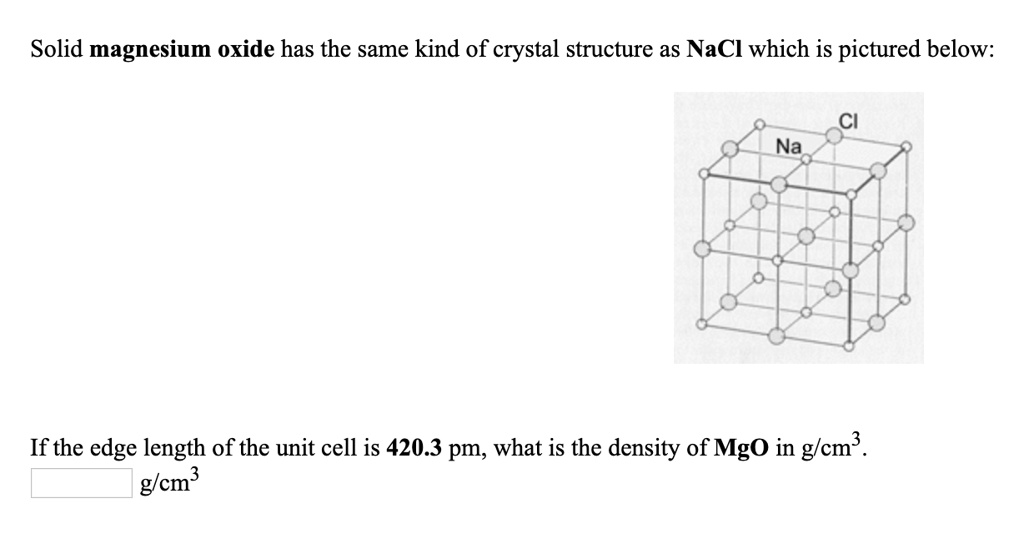
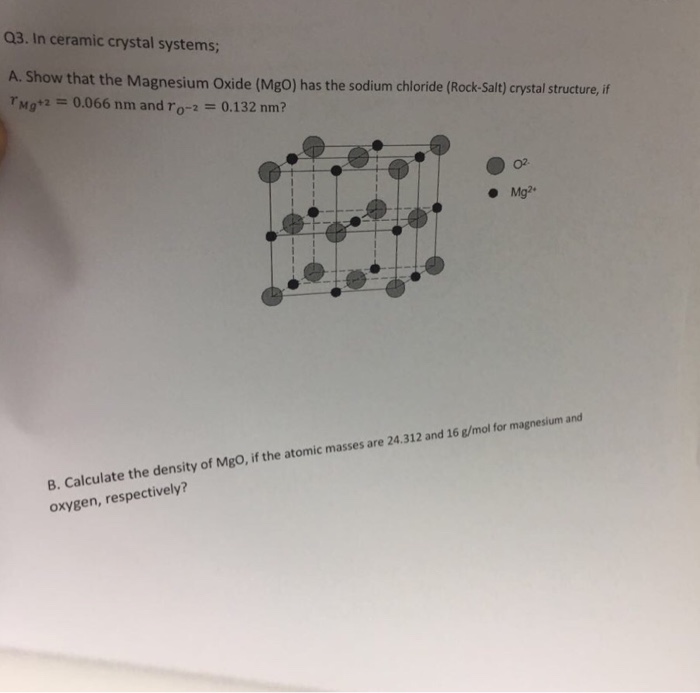
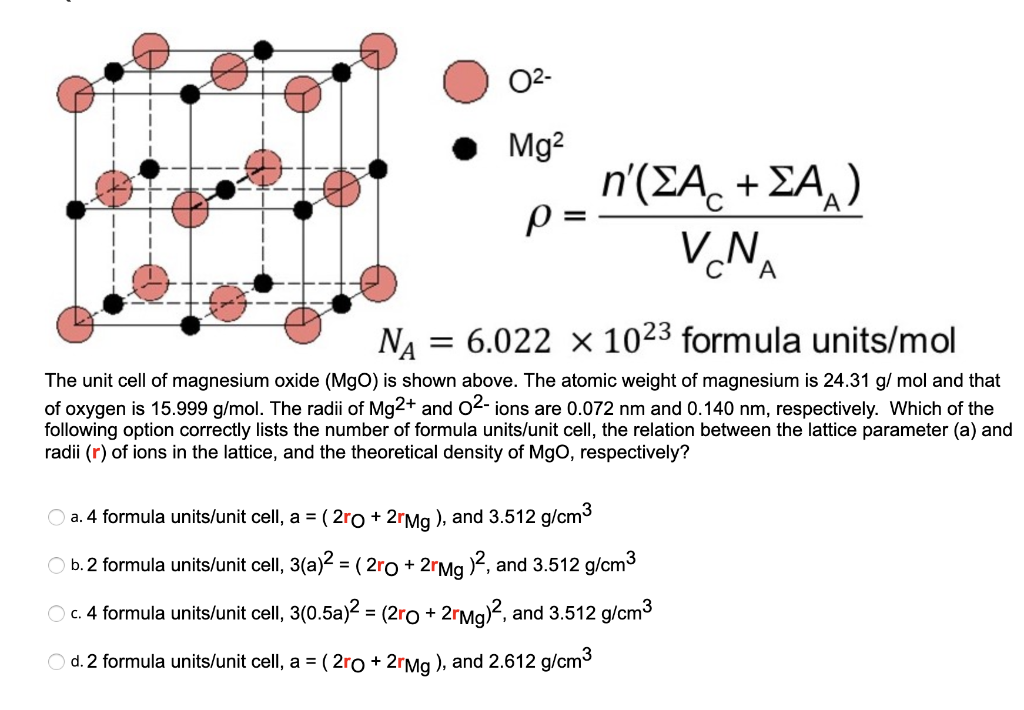
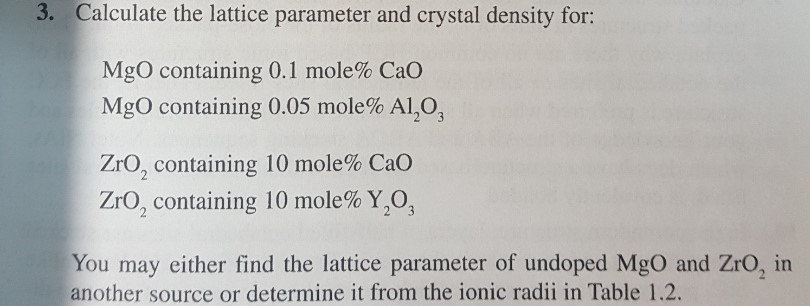
OneClass: In ceramic crystal systems; Show that the Magnesium Oxide (MgO) has the sodium chloride (Ro...

OneClass: In ceramic crystal systems; Show that the Magnesium Oxide (MgO) has the sodium chloride (Ro...

Calculated density of states (DOS) for MgO. Given as all of Mg and O... | Download Scientific Diagram
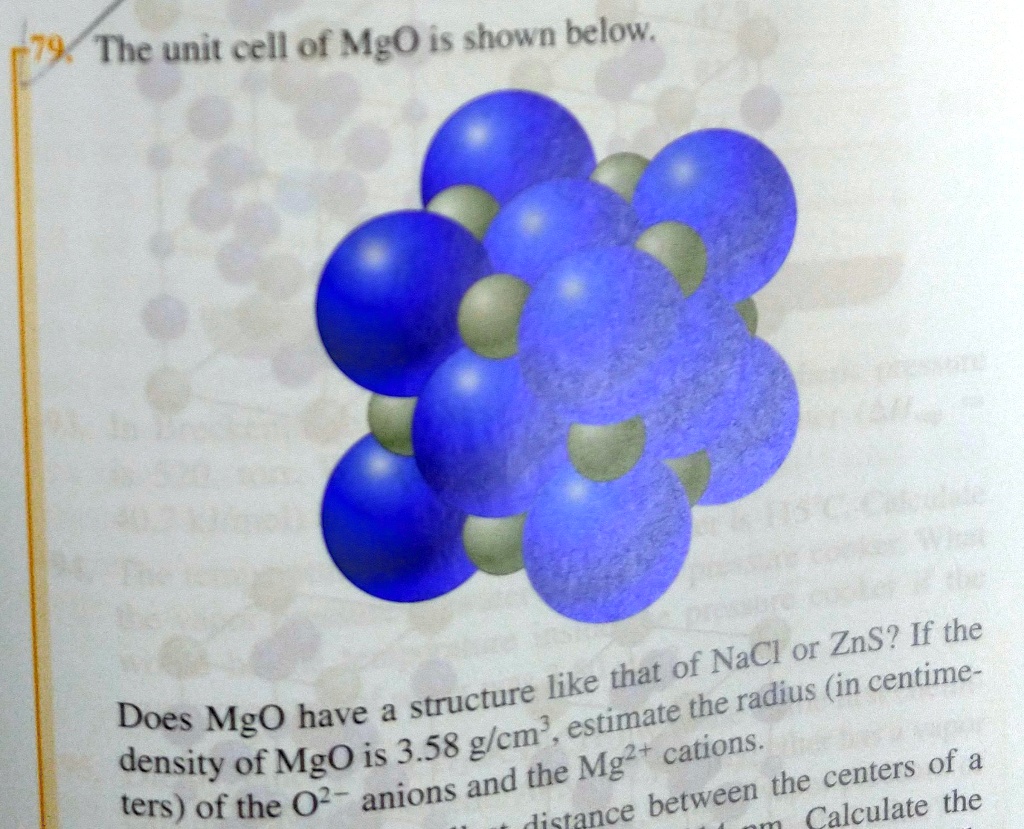
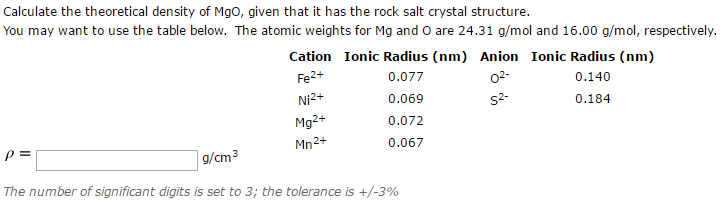
SOLVED: The unit cell of MgO is shown below; ZnS? If the NaCl or like that of (in centime- structure the radius Does MgO have estimate 3.58 glcm, density of MgO is
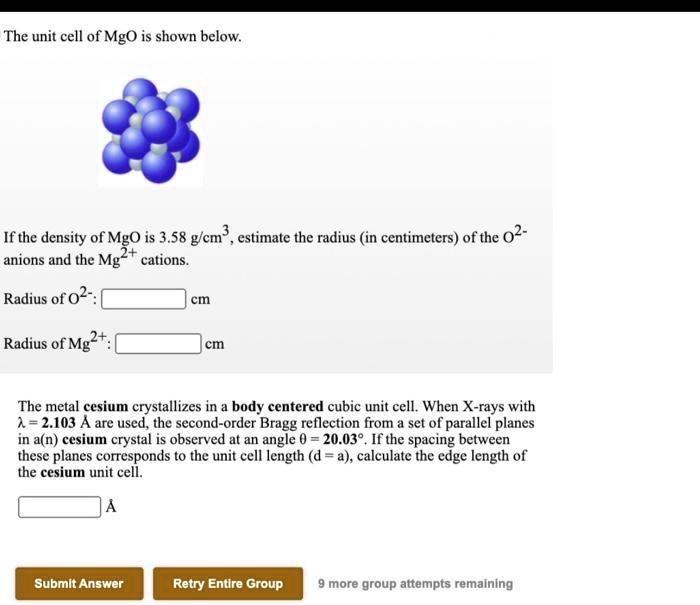
SOLVED: The unit cell of MgO is shown below: Ifthe density of MgO is 3.58 gem? estimate the radius (in centimeters) of the 02 anions and the Mg? cations. Radius of02 cm

Shock pressure–density compressibility relationship of MgO up to 1 TPa.... | Download Scientific Diagram