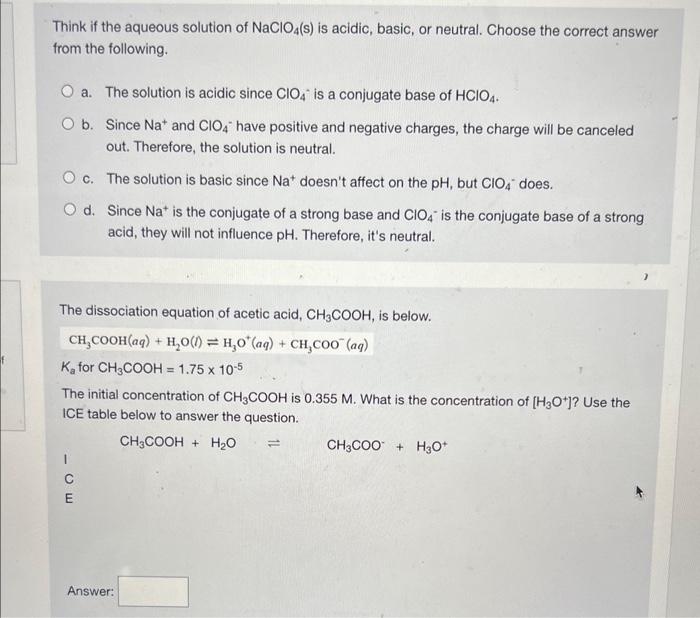
Acid (a) and base (b) titration data of 100 mg Na-AS solid in 50 ml... | Download Scientific Diagram
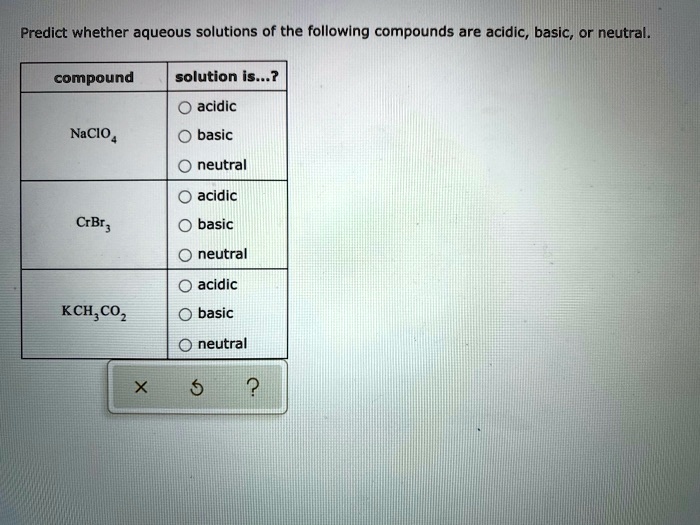
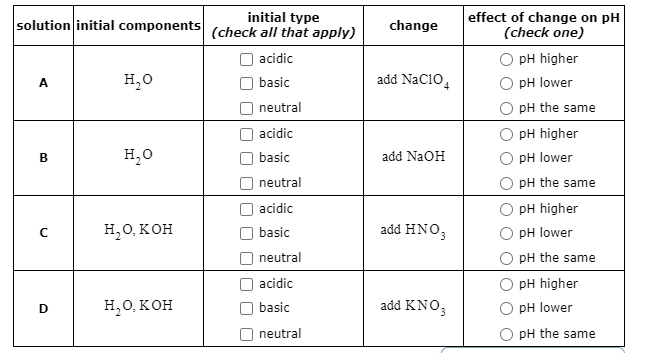
SOLVED: Predict whether aqueous solutions of the following compounds are acidic, basic, Or neutral. compound solution is...? acidic NaCIO basic neutral acidic CrBr basic neutral acidic KCH,COz basic neutral

A). Acid-base titration of raw MWCNTs I=0.01M NaClO 4 and T=20 ºC. The... | Download Scientific Diagram

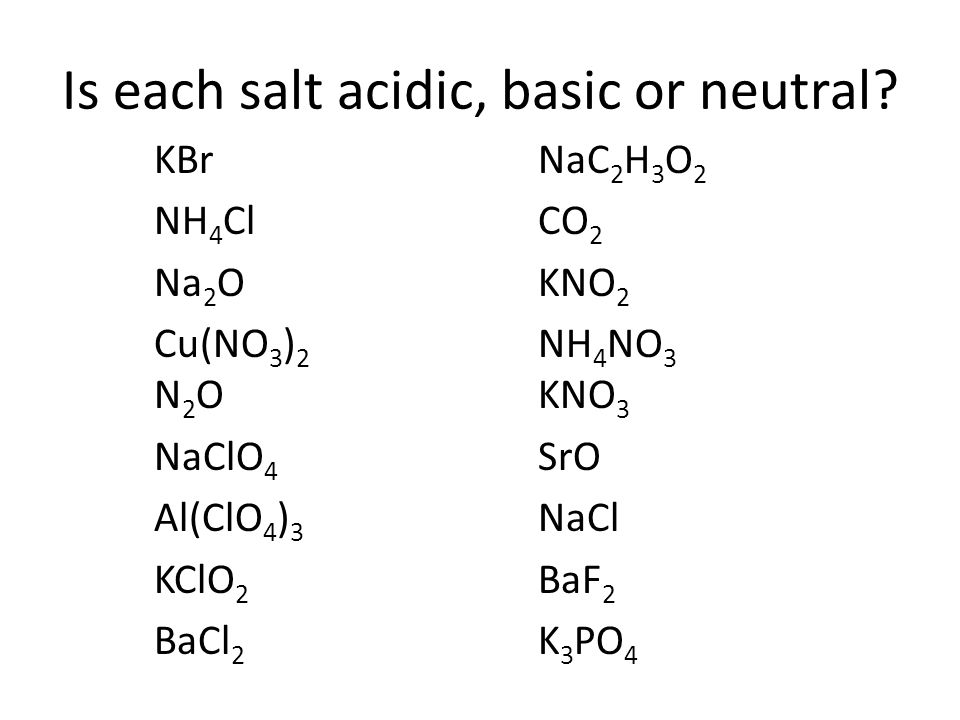
Acids, Bases, and Salts You should be able to Understand the acid-base theories of Arrhenius, Brønsted-Lowry, and Lewis. Identify strong acids and. - ppt download