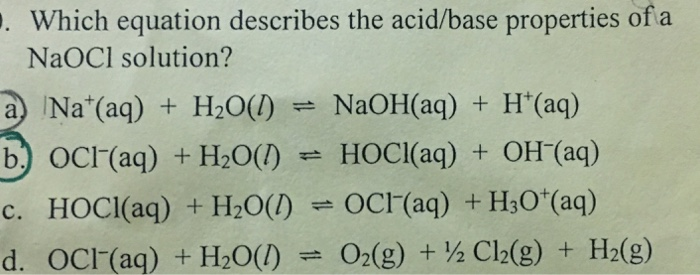Effect of sodium hypochlorite on adhesive charactersitics of dentin: A systematic review of laboratory-based testing - ScienceDirect
SciELO - Brazil - Mechanism of action of sodium hypochlorite Mechanism of action of sodium hypochlorite
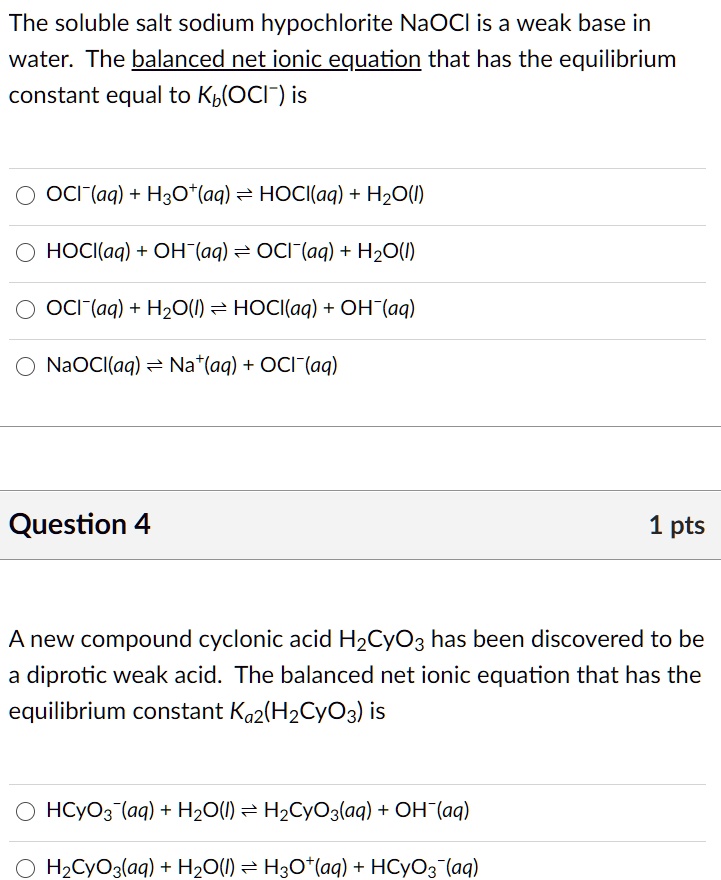
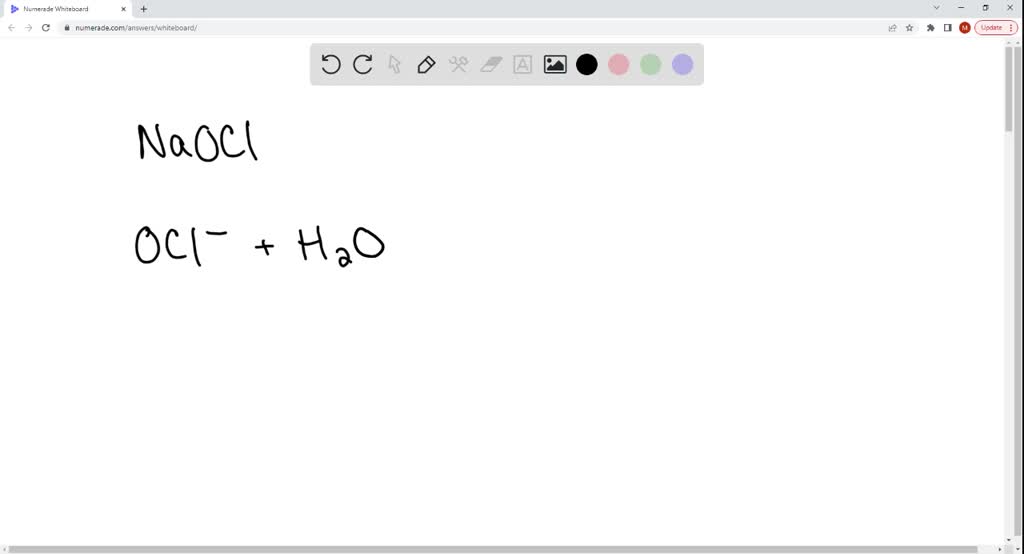
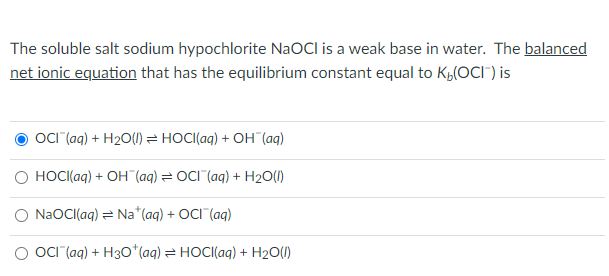
SOLVED: The soluble salt sodium hypochlorite NaOCl is a weak base in water. The balanced net ionic equation that has the equilibrium constant equal to Kb(OCl-) is: OCl-(aq) + H2O(l) = HOCl(aq) +

Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis | Organic Process Research & Development

Dissociation of chloramine-T and NaOCl. (A) In the presence of water... | Download Scientific Diagram

Aqueous solution of sodium hypochlorite (NaOCl) is a household bleach and a strong oxidizing agen... - YouTube

Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis | Organic Process Research & Development

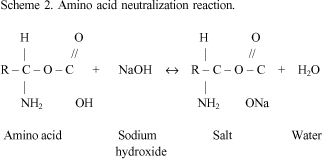
SOLVED: Match the following salts with the acid-base neutralization reactants that would produce them: A. NaClO4 = HCl + NaOH B. NaOCl = HCl + NaOH C. Ca(OCl)2 = HCl + Ca(OH)2