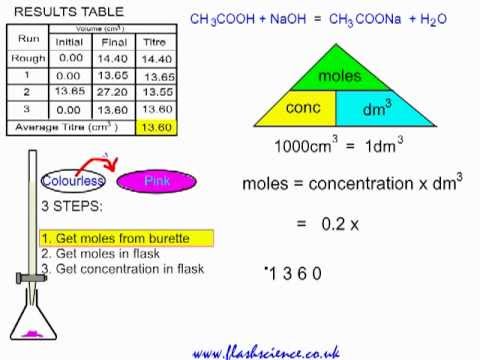
Question Video: Calculating the Concentration of a Hydrochloric Acid Solution Using Experimental Data | Nagwa

How to Calculate Molarity- With Tricks मोलरिटी आसान ट्रिक्स GPAT-NIPER-Pharmacist Exam | Model question paper, Question paper, Online tests

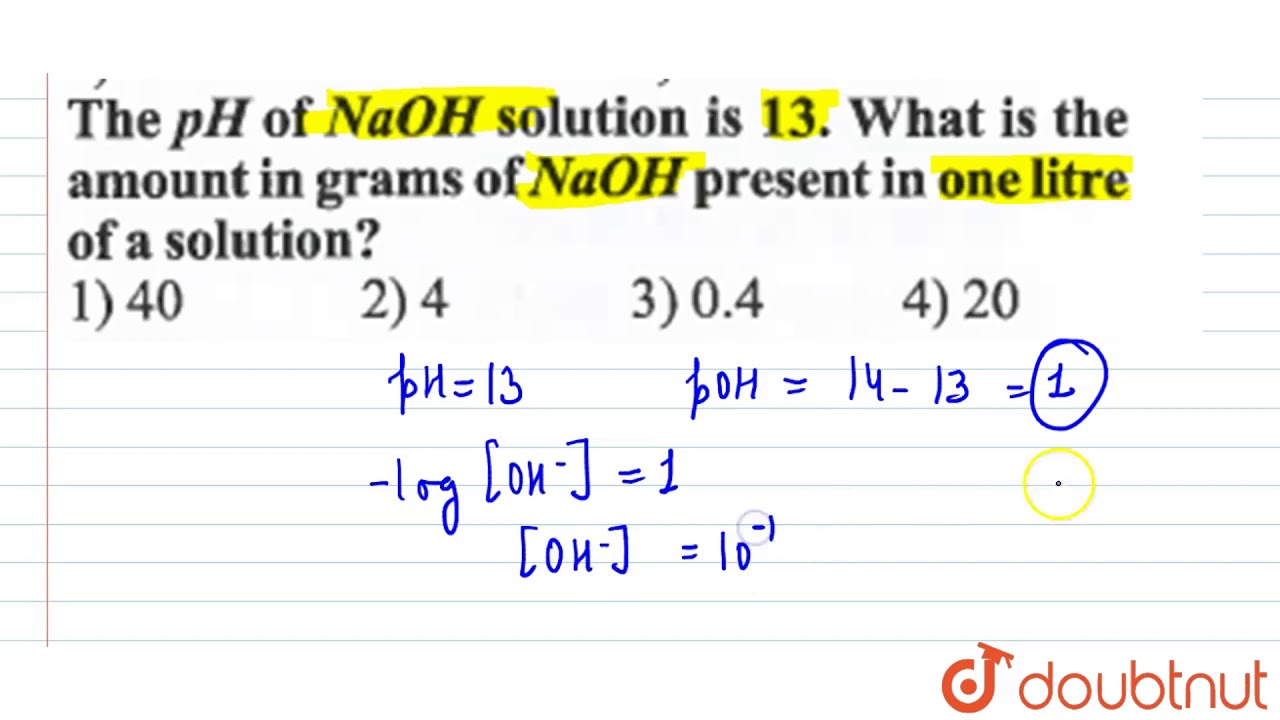
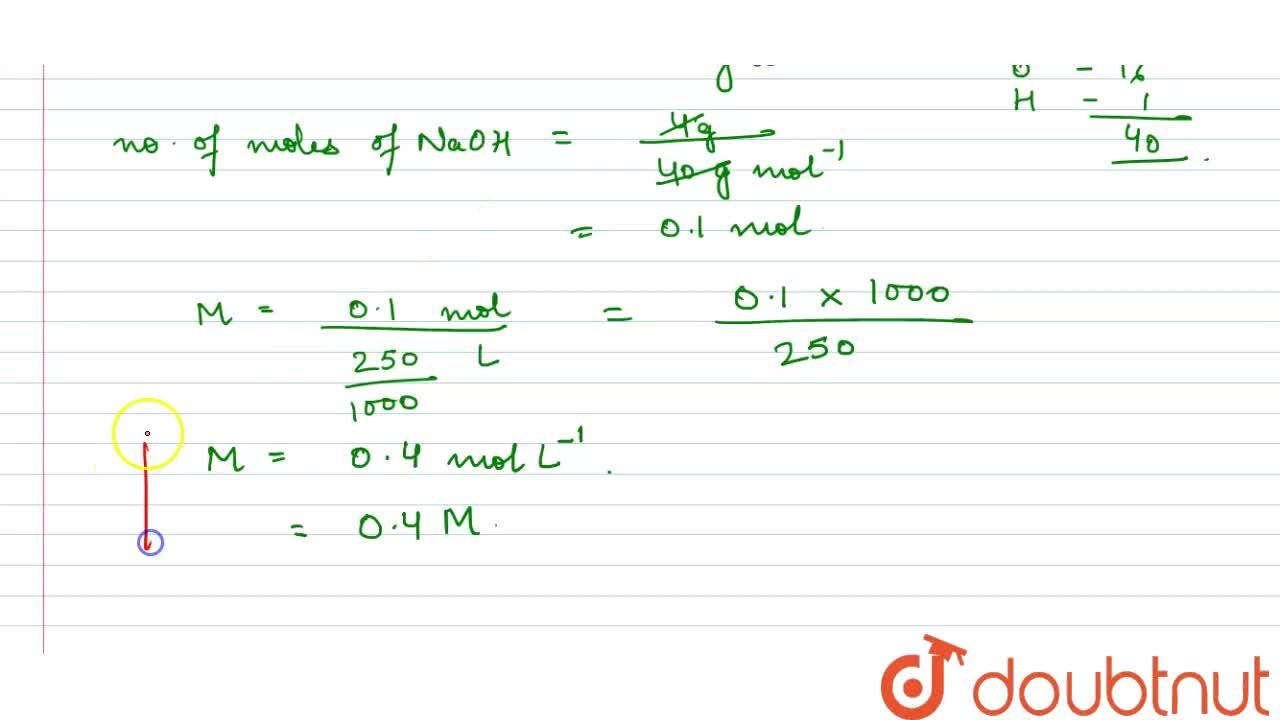
Molarity of NaOH in a solution prepared by dissolving 4 g of NaOH in enough water to form 250 ml of solution is:A. 0.4 MB. 4.1 MC. 1.4 MD. 0.45 M

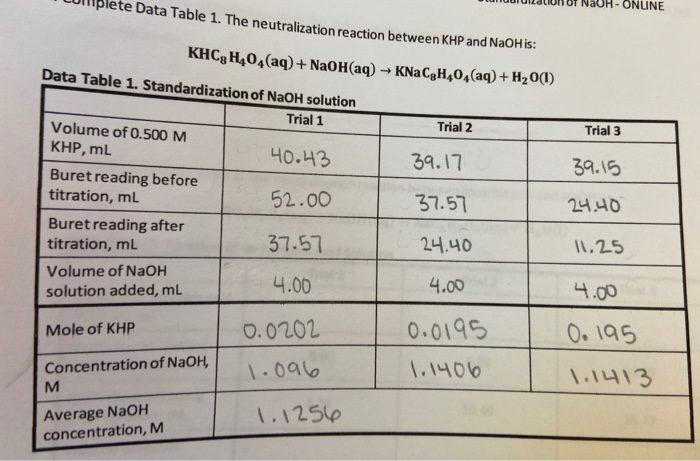
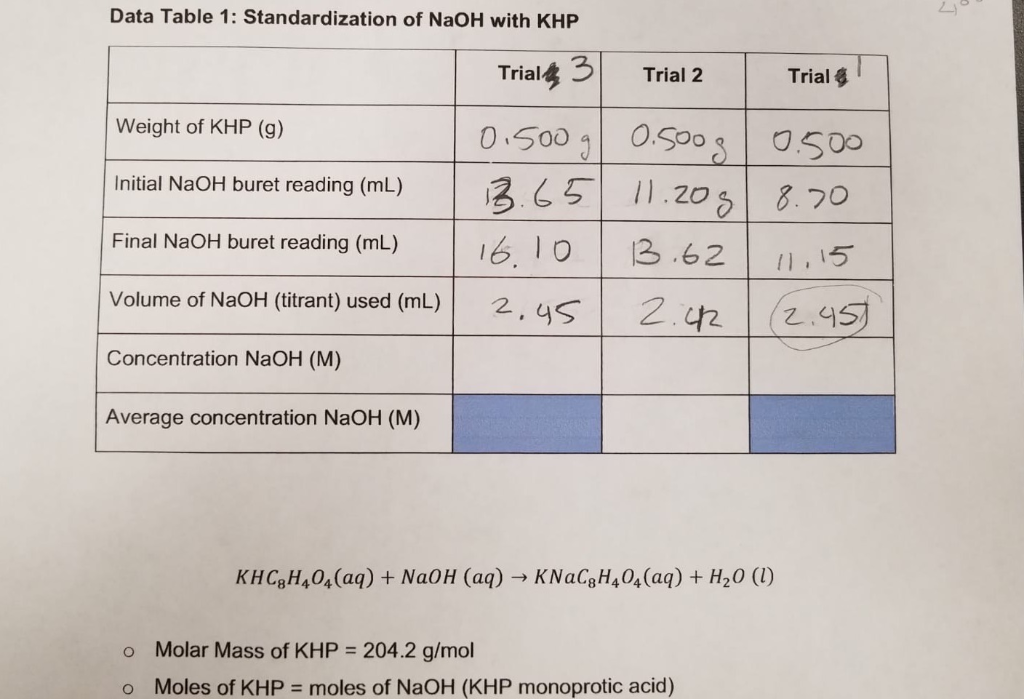
SOLVED: Calculations and questions (15points) Standardization of NaOH Volume of NaOH used 18.3mL 18.6 mL -17.8 mL Trial #1 Trial #2 Trial #3 (if > 3 trials, indicate additional volume(s) On other

Calculate molarity of NaOH in a solution made by mixing 2 L of `1.5 M NaOH,3 L` of 2 M NaOH and 1L - YouTube

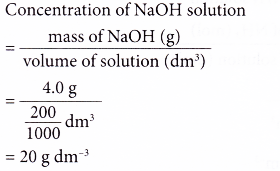
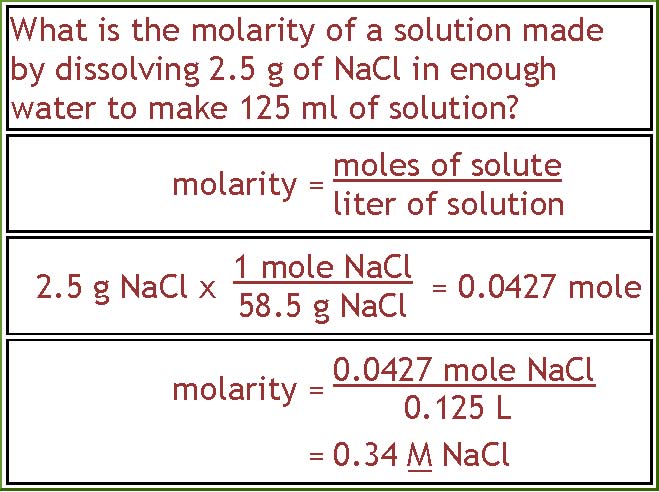
Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to... - YouTube

Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility gcse chemistry igcse KS4 science A level GCE AS A2
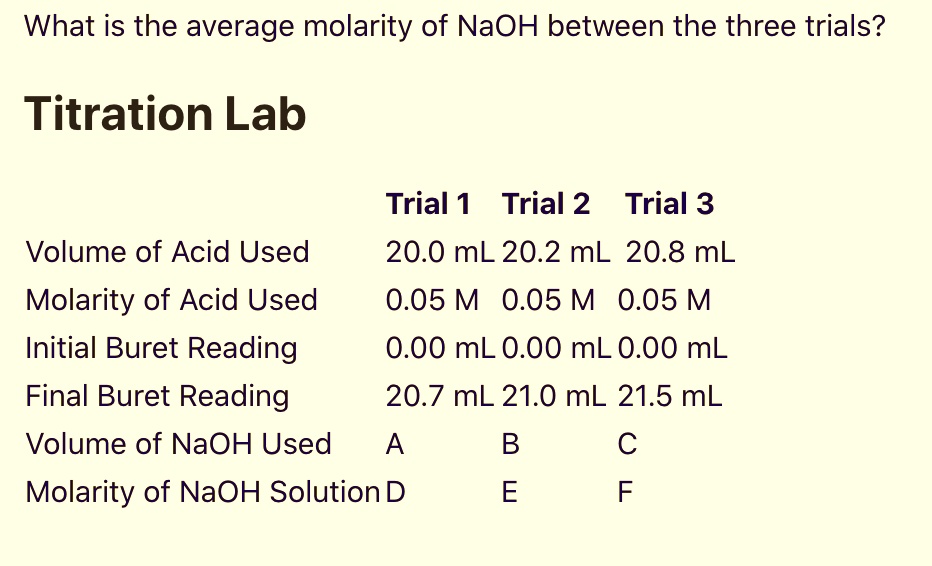
SOLVED: What is the average molarity of NaOH between the three trials? Titration Lab Trial 1 Trial 2 Trial 3 20.0 mL 20.2 mL 20.8 mL 0.05 M 0.05 M 0.05 M
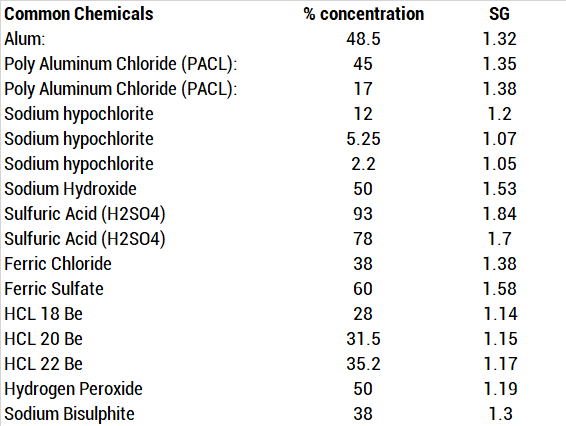
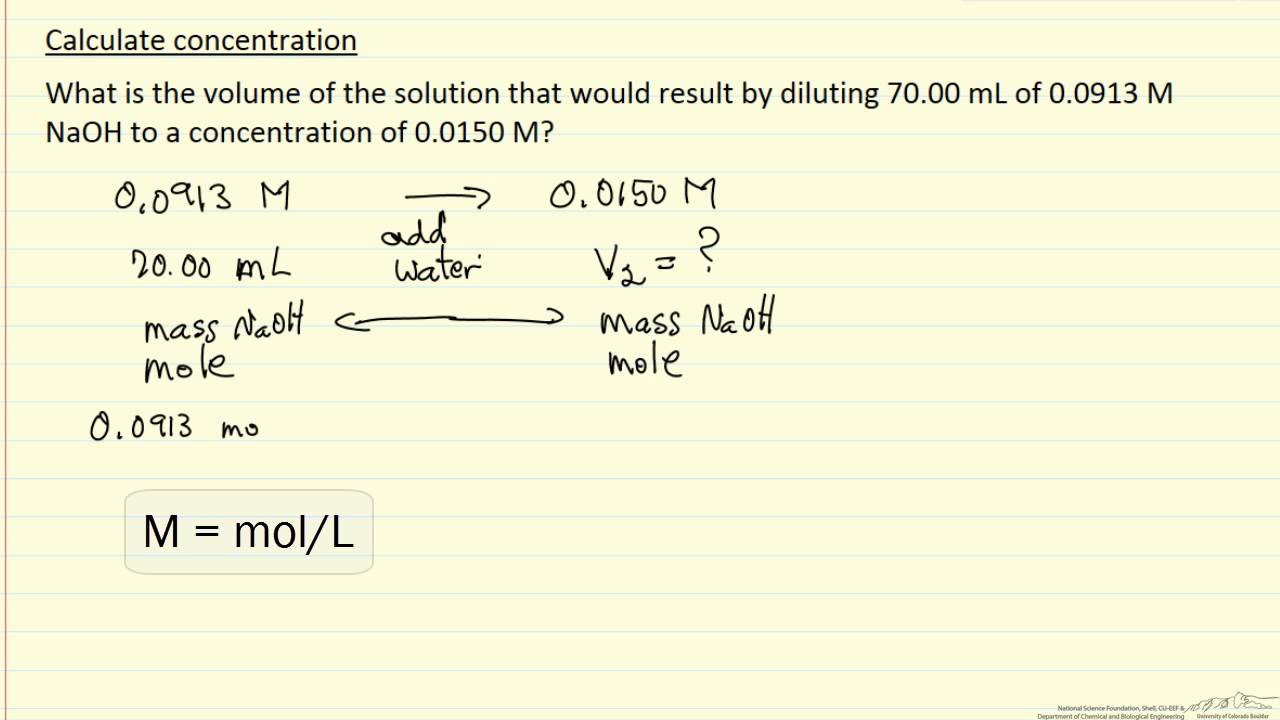
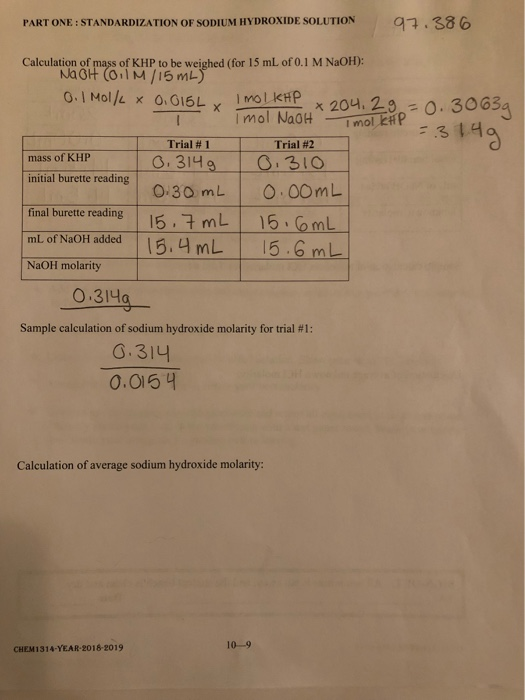
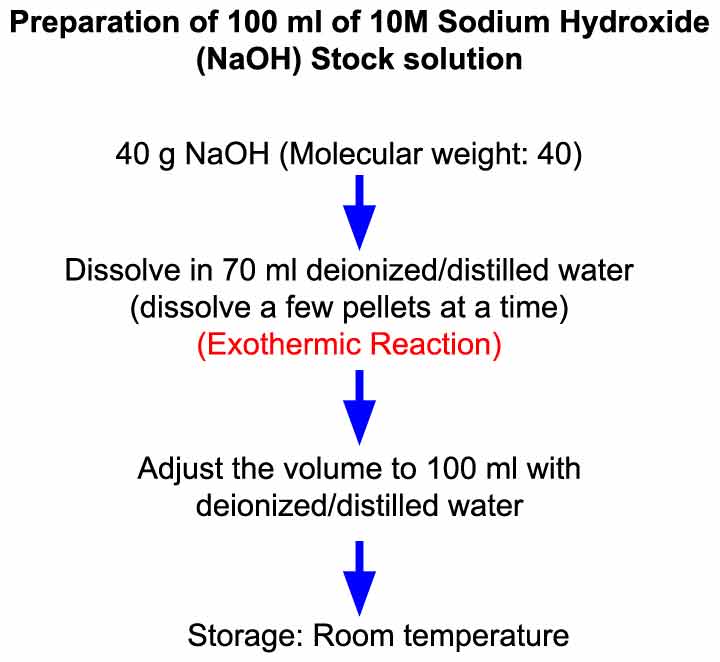




:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)