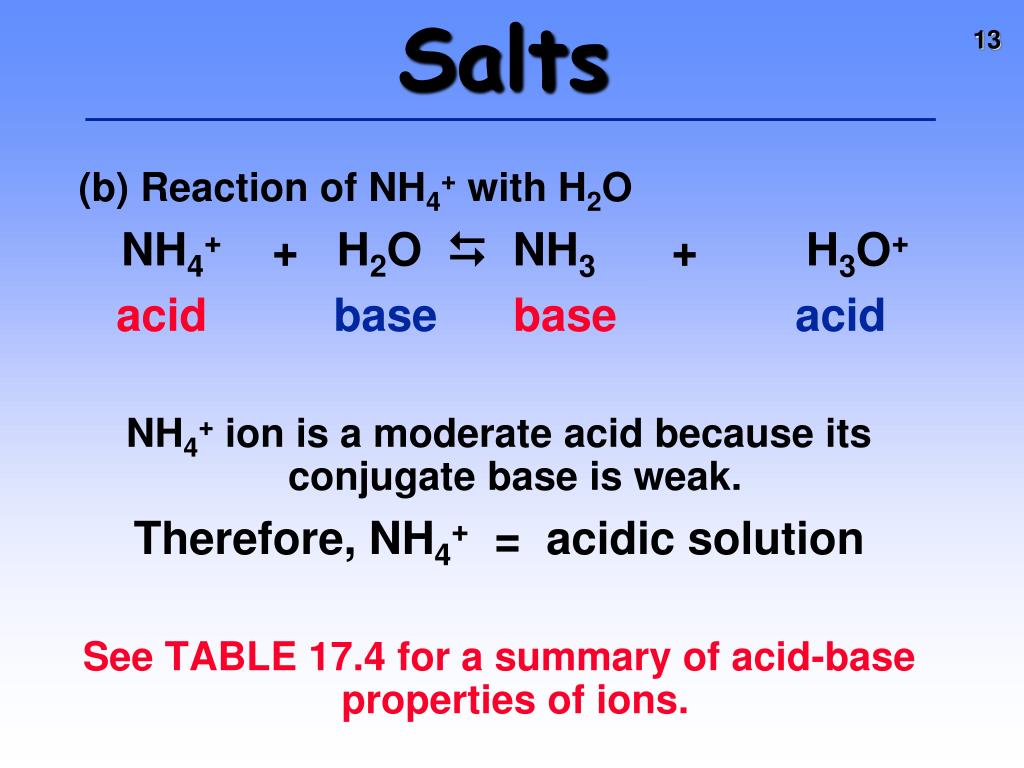
⚗️HELP In the following acid-base reaction, NH4+ is the H2PO4- (aq) + NH3(aq) → HPO42- (aq) + - Brainly.com
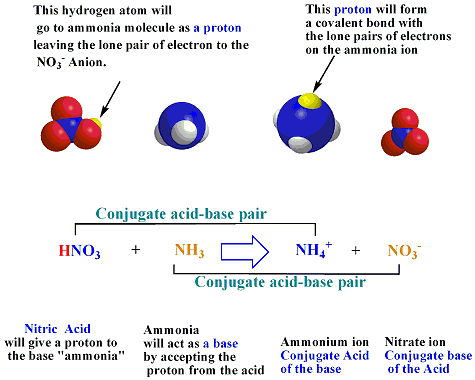
In the following acid-base reaction, how would you identify the acid, base, and their conjugate acids and bases: NH4+ + HCO3- --> NH3 + H2CO3? | Socratic

Role of NH3 and NH4+ transporters in renal acid-base transport | American Journal of Physiology-Renal Physiology
Why is NH4+ a weak acid? I have learned that the base and it's conjugate acid is opposite in strength, so why is NH4+ a weak acid when NH3 is also a

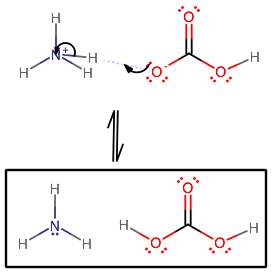
Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com

SOLVED: In the reaction NH3 (aq) + H2O (I) <–> NH4+ (aq) + OH- (aq), which is the conjugate acid-base pair? OH- NH3 NHA+, OH- NH3, H2O NH4+, NH3