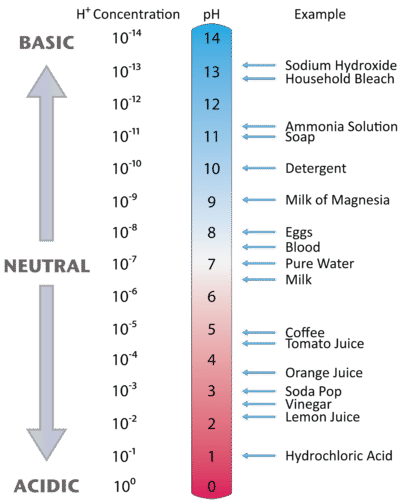
The pH scale The pH scale is used to measure how acidic or basic a solution is. The lower the pH, the more acidic the solution. The higher the pH, the. -
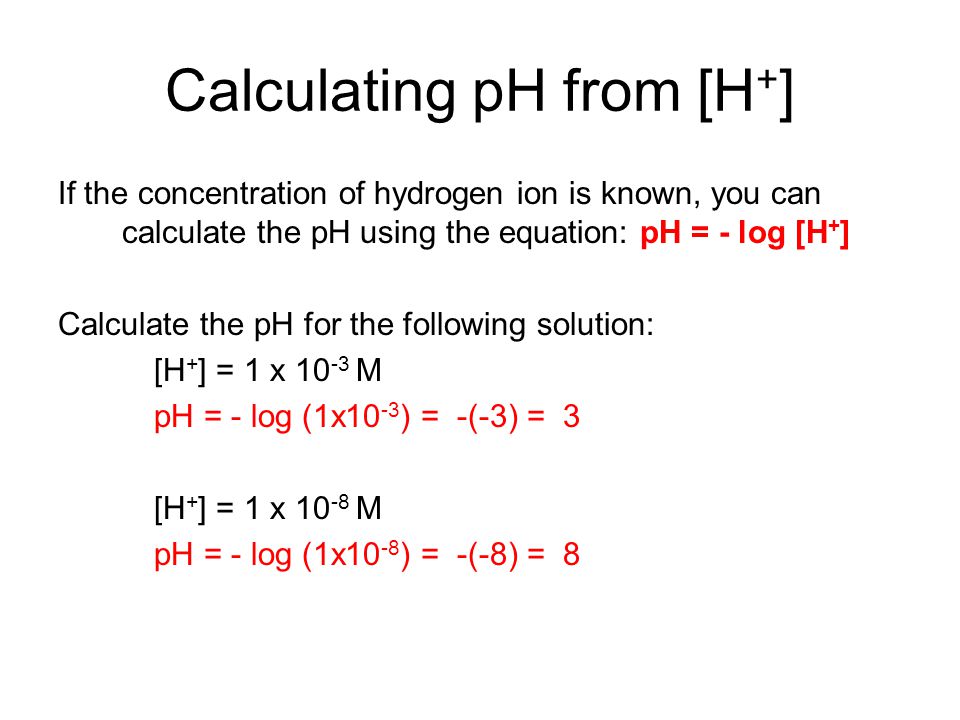
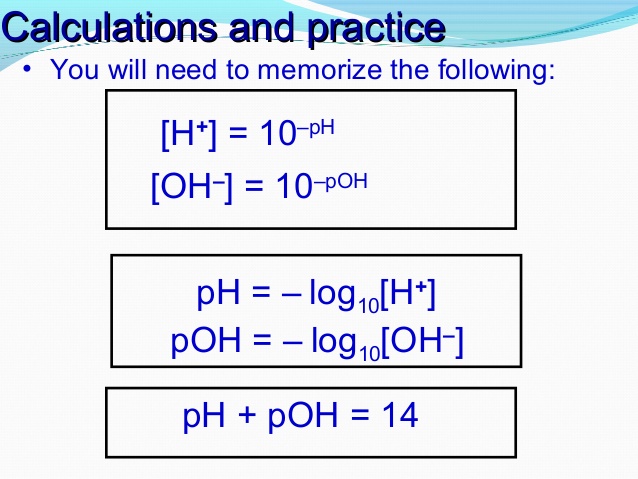
The pH of a solution describes its acidity and is the negative logarithm ( log) of its hydrogen ion concentration. The term pH is used because the hydrogen ion concentration in solutions of weak acids and in many other fluids is frequently much less than 1 ...
![SOLVED: Acidic or basic solution, pH The pH of a solution is the negative logarithm of the hydrogen-jon concentration: pH = #log[h"] log stands for logarithm to the base 10 log 100 = SOLVED: Acidic or basic solution, pH The pH of a solution is the negative logarithm of the hydrogen-jon concentration: pH = #log[h"] log stands for logarithm to the base 10 log 100 =](https://cdn.numerade.com/ask_images/54471c304890403dbf6cbad11744b807.jpg)
SOLVED: Acidic or basic solution, pH The pH of a solution is the negative logarithm of the hydrogen-jon concentration: pH = #log[h"] log stands for logarithm to the base 10 log 100 =
![pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarit… | Chemistry lessons, Chemistry education, Chemistry classroom pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarit… | Chemistry lessons, Chemistry education, Chemistry classroom](https://i.pinimg.com/736x/99/80/05/998005d7b3fbb74f7a91222f3209e7c5--physical-chemistry-ap-chemistry.jpg)
![Solving For pH, pOH, [H+], [OH-] - Acids & Bases Solving For pH, pOH, [H+], [OH-] - Acids & Bases](http://youarebasic.weebly.com/uploads/5/0/1/4/50143245/5802360_orig.png)
:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)
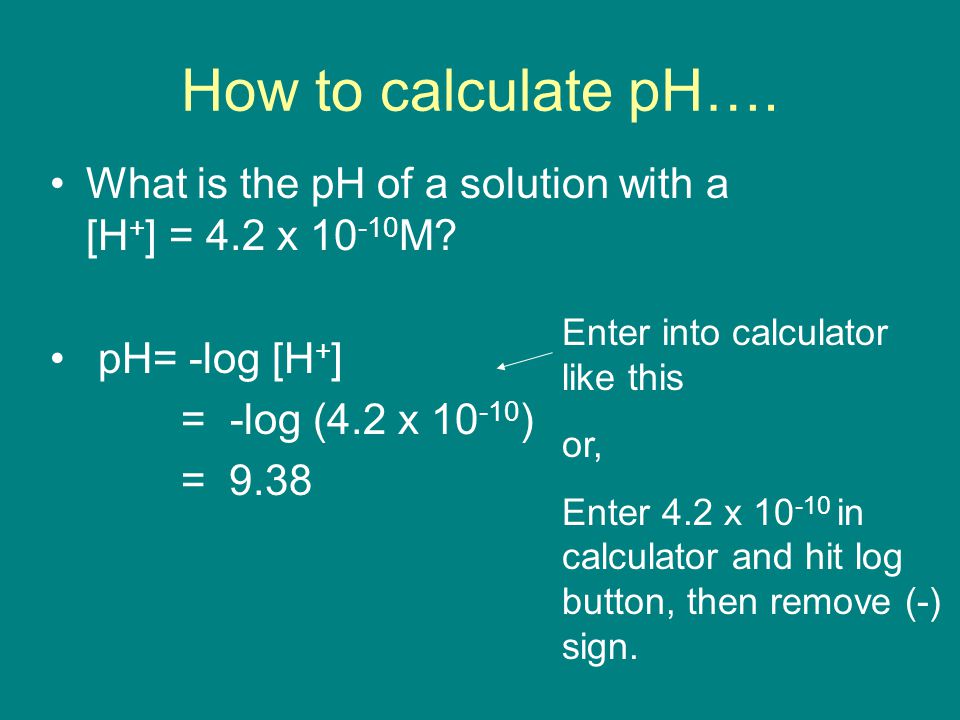



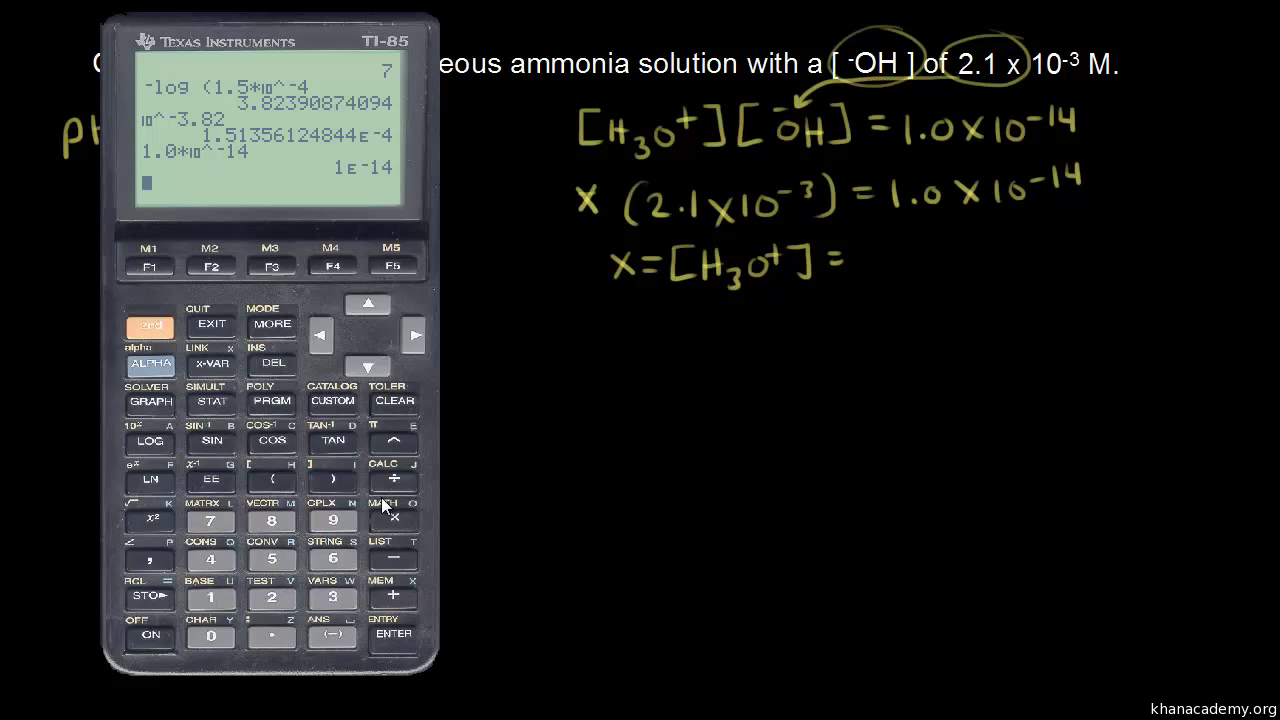

![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)
![Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC" Finding the pH, pOH, [H+], [OH-] - ACIDS AND BASES: IT'S ACTUALLY Quite "BASIC"](http://itsactuallyquitebasic.weebly.com/uploads/2/7/8/0/27808159/4950515.png?357)





![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)