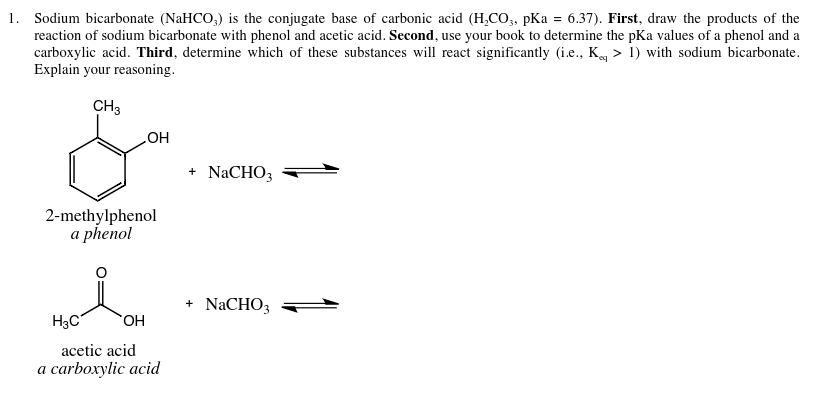
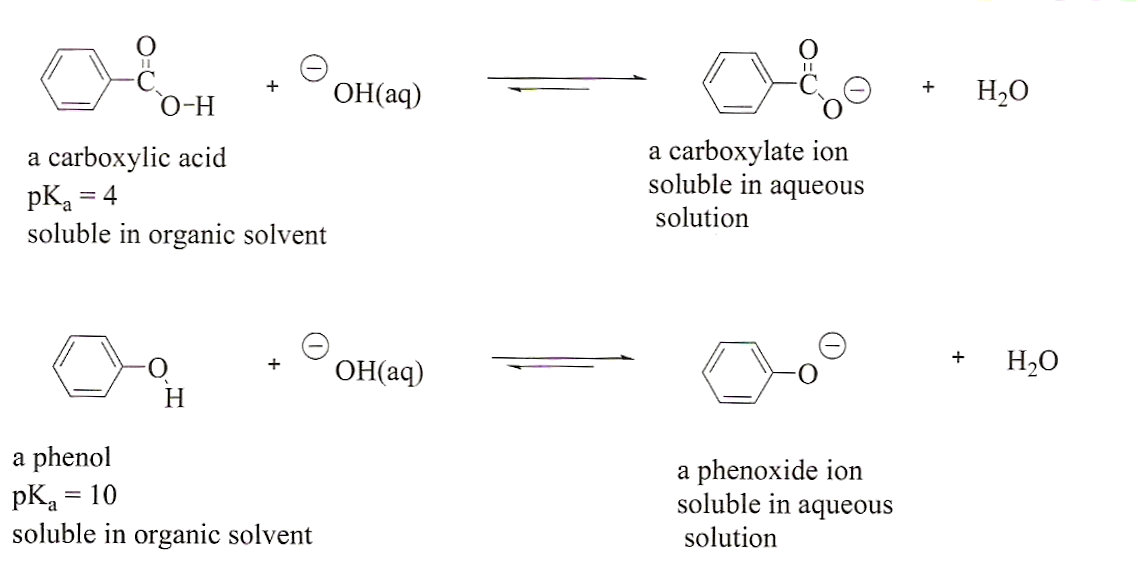
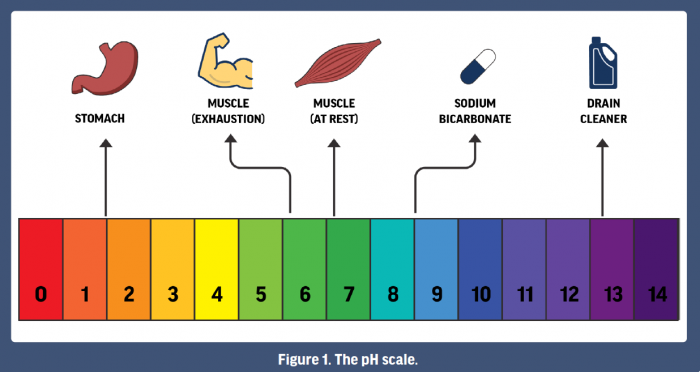
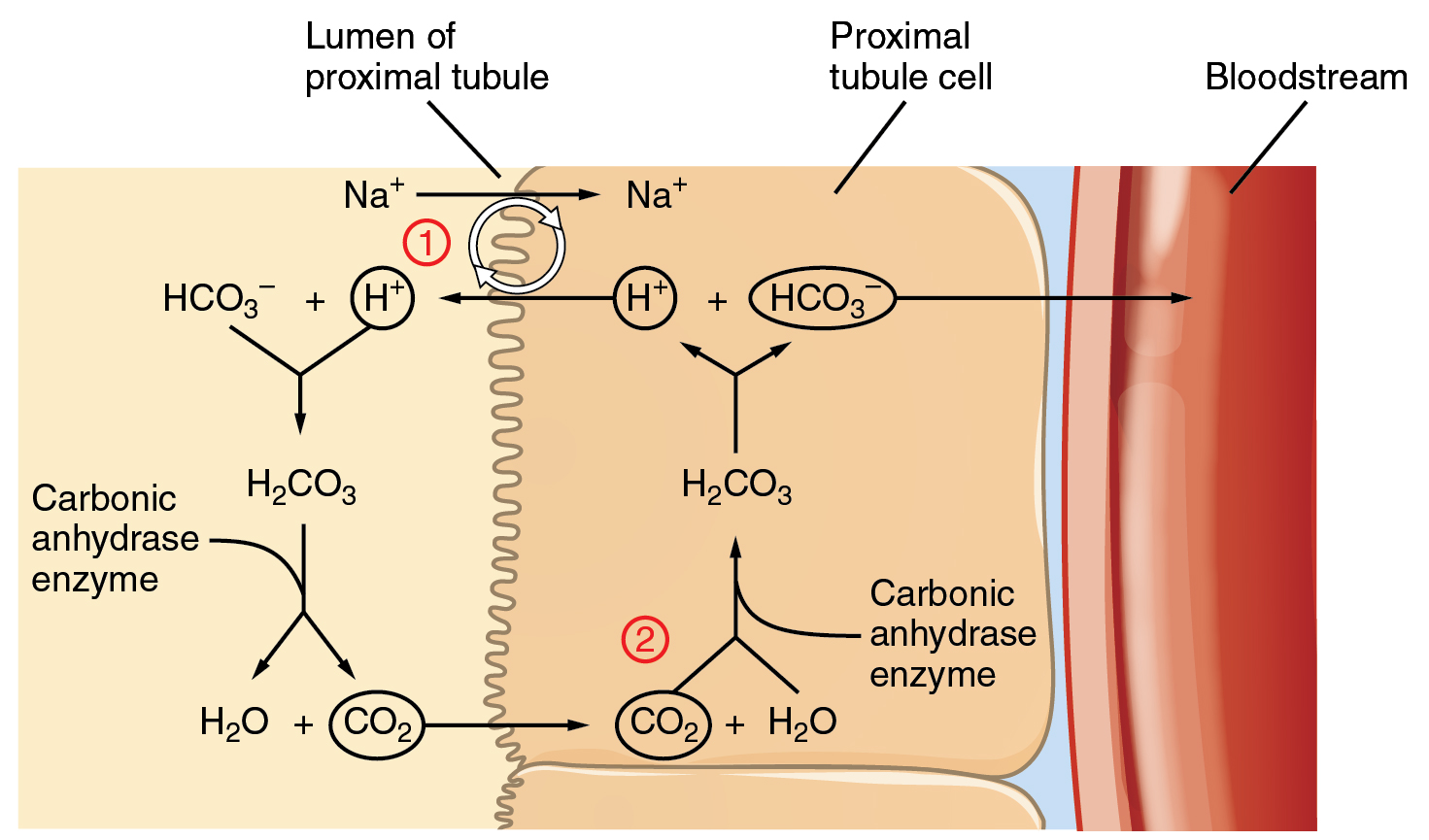
Question Video: Identifying the Salt Product of the Reaction between Ethanoic Acid and Sodium Bicarbonate | Nagwa
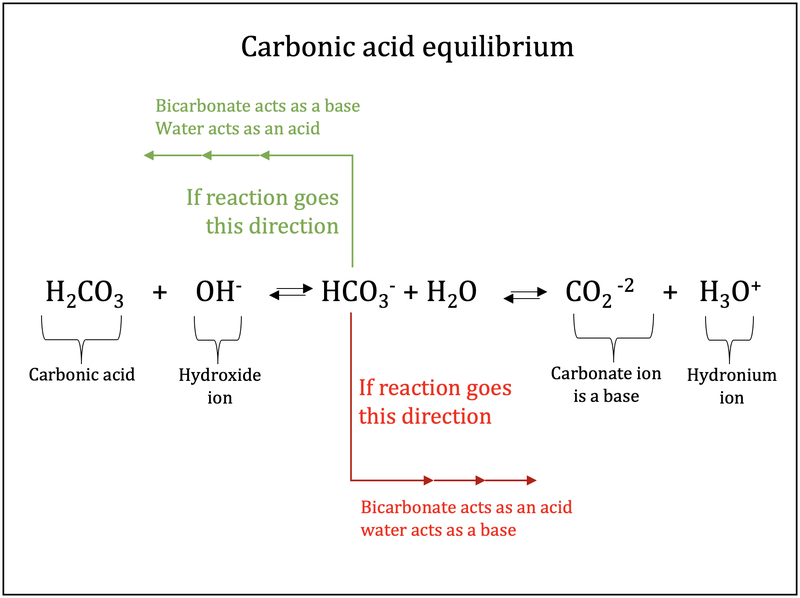
72.Baking soda is an acidic salt or a basic salt.? (NaHCO3 is formed from a weak acid and a strong base so it should be basic but it also has one replaceable

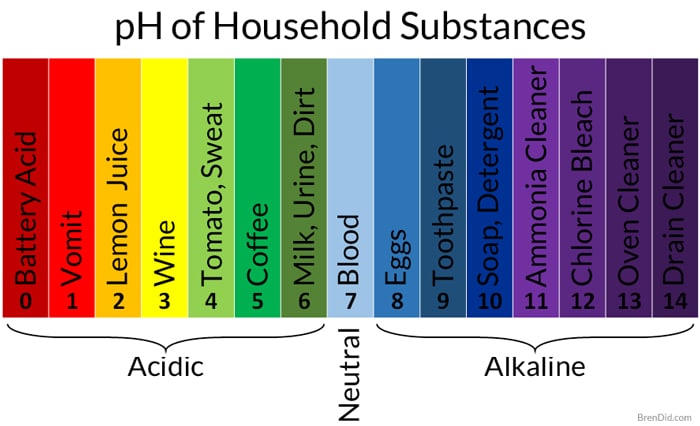
Acids and Bases Acids taste sour (citric acid, acetic acid) Bases taste bitter (sodium bicarbonate) There are 3 ways to define acids and bases, you will. - ppt download







![MCQ] Sodium hydrogen carbonate when added to acetic acid evolves gas MCQ] Sodium hydrogen carbonate when added to acetic acid evolves gas](https://d1avenlh0i1xmr.cloudfront.net/24d7669e-1012-43e2-9ede-446e6d9b029d/reaction-of-sodium-hydrogen-carbonate-with-acetic-acid-01.jpg)