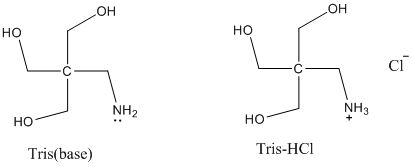
Solved: Chapter 17 Problem 92AP Solution | Loose Leaf Version For Chemistry: Atoms First 2nd Edition | Chegg.com

New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions - ScienceDirect
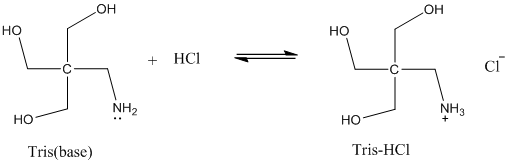
Solved: Chapter 17 Problem 92AP Solution | Loose Leaf Version For Chemistry: Atoms First 2nd Edition | Chegg.com

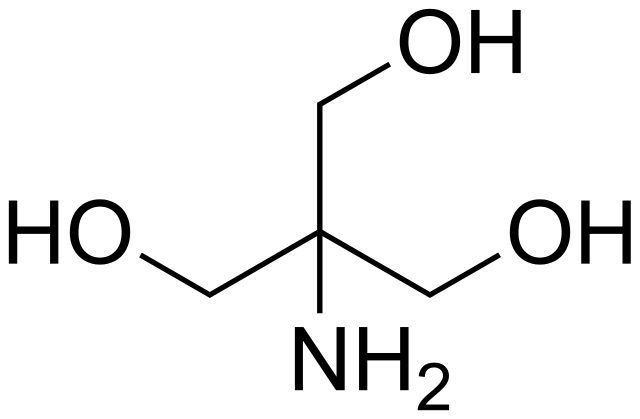
Scheme 2. Structures of Tris, TrisHCl and etilendiaminotetraacetic acid (H4EDTA) : pH and Acid-Base Equilibrium Calculations via a Matrix Representation of Solutions of Acids and/or Bases : Science and Education Publishing
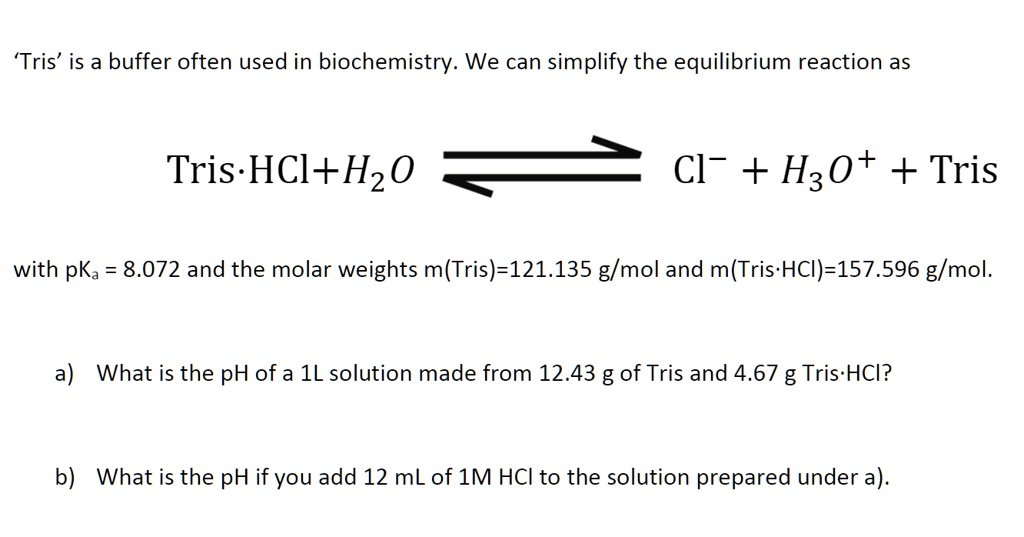






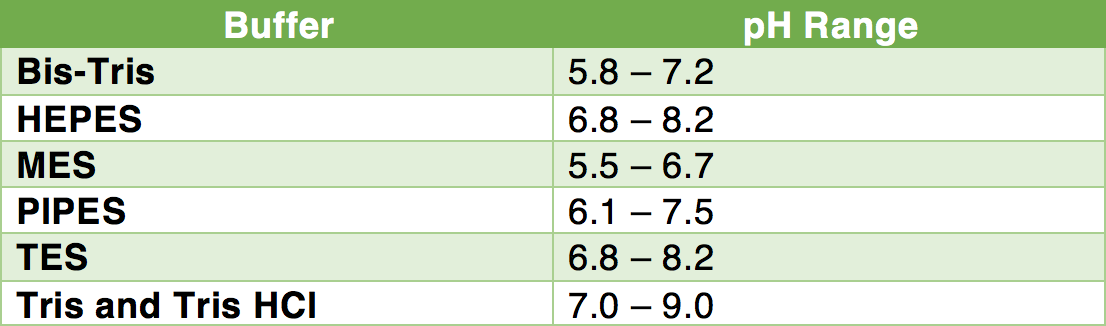






![T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60050-500.0.jpg)


![BT019b] 1M Tris-HCl, pH 8.0 | Biosolution BT019b] 1M Tris-HCl, pH 8.0 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT016-1M-Tris-HCl.jpg)